
- 349 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Structure
About this book
This textbook summarizes physical aspects of materials at atomic and molecular level, and discusses micro-structure of metals, alloys, ceramics and polymers. It further explains point defects, dislocations and surface imperfections, and the motions of atoms and molecular in solid state. As first volume in the set, it prepares students for further studies on phases and transitions which are discussed in the next volume.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Edition
1Subtopic
Atomic & Molecular PhysicsChapter 1 Atomic structure and interatomic bonding
Material is referred to a substance with present or expected future applications for human beings and has been considered as the substantial basis of the national economy. The development of industrial and agricultural production, the science and technology progress, and the improvement of people’s living standards are relying on an extensive sort of materials, such as metals, ceramics, and polymers, having various properties that meet the different requirements. For a long time, people have been studying and investigating the factors that influence the properties of the materials and the ways to improve their performance when using the materials. The researches have shown that the atomic structure of the elements within the material is the fundamental factor that determines the material performance. It includes interactions and combinations among atoms, the spatial distribution and movement of atoms or molecules, and the morphology of atomic clusters and so on. Therefore, the first topic we need to understand is the microscopic structure of the material and its internal construction and microstructure state, so that we can find out ways to improve and develop new materials from its internal contradictions.
A substance is made up of atoms, and an atoms consist of a positively charged nucleus is located in the center of the atom and negative electrons outside the nucleus.
The electronic structure of atoms determines the interatomic bonding of the atoms with each other. Therefore, the understanding of the electronic structure of atoms is not only helpful to classify the materials, but also useful to find out the fundamental principles of physical, chemical, and mechanical properties of materials.
1.1 Atomic structure
1.1.1 Substance construction
As is known to all material is composed of numerous fine particles gathered together in some certain way. These particles may be considered as molecules, atoms, or ions. The molecule is a kind of particle that can exist alone and maintain its chemical properties. The volume of a molecule is small, for example, the diameter of a water molecule is about 0.2 nm. However, the mass of different molecules is different. For instance, H2 is the smallest molecule in the world and its relative molecular mass is only 2; however, a natural polymer compound, such as proteins, has an average relative molecular weight of up to a few millions.
Further analysis showed that the molecule is composed of a number of smaller particles, i.e., atoms. In a chemical reaction, the molecules can be further broken down into the atoms; whereas, the atoms are indivisible. Therefore, an atom is the smallest particle in a chemical reaction. However, the atoms are not the basic particles of the material in quantum mechanics. They have complex structures. The atomic structure directly affects the interatomic bonding.
1.1.2 Structures of atoms
The recent scientific experiments show that the atom is composed of the protons and neutrons, as well as the electrons. The neutron in the nucleus is electrically neutral and the proton carries a positive charge, which possesses exactly the same charge as that of an electron, whose charge is equal to –e (e = 1.6022 × 10−19 C). The electrostatic attraction shows that the negatively charged electrons are tightly bound around the positively charged nucleus. As a whole, an atom is electrically neutral because in an atom the number of protons and electrons is the same.
An atom is volumetrically small. The atomic diameter is about the order of 10−10 m, while the diameter of nucleus is only about the order of 10−14 m. Besides, the atom mass is mainly undertaken by the nucleus, while the surrounded electrons are located within a relatively enormously large space in an atom. The mass of an neutron or an proton is approximately 1.67 × 10−24 g, while the mass of electron is about 9.11 × 10−28 g; thus a proton is almost 1,833 times an electron.
1.1.3 Electronic Structures of atoms
The electrons revolve around the nucleus of an atom, which is just like a “cloud” with a negative charge around the nucleus, so it is called the electronic cloud. The electrons have the duality of the wave and particle. The electronic motion doesn’t have fixed orbits but the position probability of its motion outside the nucleus can be determined through the statistical methods according to the energy levels of electrons. The electrons with a lowest energy usually appear near the nucleus, while the electrons with the highest energy move far away from the nucleus. In quantum mechanics, the basic equation reflecting the motion of the microscopic particles is the Schrodinger’s equation. The wave function obtained by solving the equation describes the state of the electronic movement and the occurrence probability in somewhere outside the nucleus. Videlicet, the spatial position and electron energy in an atom can be determined by the four quantum numbers shown as follows:
(1) Principal quantum number n
It determines the electron energy and its average distance with the nucleus of an atom, i.e., the quantum shell of the electron, as shown in Fig. 1.1. n can only be a positive integer, such as 1, 2, 3, 4,…, and it explicitly represents the quantization of the electron energy in an atom, thus being called the principal quantum number. The principal quantum number (or quantum shell) is represented by a capital letter. For example, n=1 means that the quantum shell has the lowest energy, and it is the closest orbit to the nucleus, named as K-shell, according to the old quantum theory. The numbers 2, 3, 4, … indicate higher energy levels named L-, M-, N-, …, respectively.
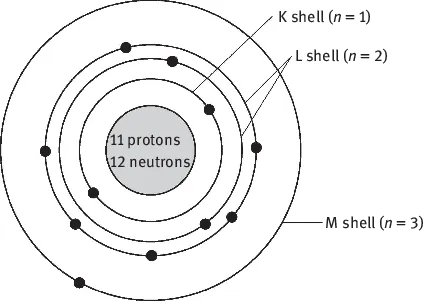
Fig. 1.1: The electron distribution of K-, L- and M-shells in the Na atomic structure.
(2) Azimuthal quantum number li
It indicates the energy level of an electron in the same quantum shell, i.e., the electron subshell. The azimuthal quantum number (also referred to as the secondary quantum number) l can be 0, 1, 2, 3, …. For example, when n equals 2, it means that two orbital azimuthal quantum numbers exist, l2 = 0 and l2 = 1, that is, L-shell consists of two electronic subshells according to the electronic energy difference. For convenience, the electron energycorresponding to the orbit with an azimuthal quantum number of li is commonly labeled as lowercase letters as follows:
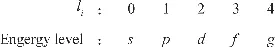
In the same quantum shell, the electron subshell energy is increased by the order of s, p, d, f, and g. The electronic cloud pattern varies in different electronic subshells. For instance, the electronic cloud of s subshell is spherical with the atomic nucleus sitting at the center, and the electronic cloud of p subshell is spindle.
(3) Magnetic quantum number mi
It gives the energy series or orbital number of each azimuthal quantum number. In each electron subshell, the total number of a magnetic quantum number is 2li + 1. If li equals 2, the magnetic quantum number is 2 × 2 + 1 = 5, with the values of −2, −1, 0, + 1, + 2.
Magnetic quantum number determines the spatial orientation of the electron cloud. If the space occupied by the electron cloud has a certain pattern and extension direction in a certain quantum shell, we name it as an orbital, then the four subshells, s, p, d, and f, will have 1, 3, 5, and 7 tracks, respectively.
(4) Spin quantum number si
It reflects different electron spin directions Si is specified as +1/2 and −1/2, which illustrate the spinning clockwise and counterclockwise and are commonly represented by “↑” and “↓”, respectively.
In the nucleus with no less than one electron, the arrangement of electrons outside the nucleus follows the three principles, i.e., the minimum energy principle, the Pauli exclusion principle, and the Hund’s rule.
(a) Minimum energy principle
The elect...
Table of contents
- Title Page
- Copyright
- Contents
- Chapter 1 Atomic structure and interatomic bonding
- Chapter 2 The structure of solids
- Chapter 3 Crystal defects
- Chapter 4 Deformation and recrystallization
- Chapter 5 Diffusion in solids
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Structure by Gengxiang Hu,Xun Cai,Yonghua Rong in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Atomic & Molecular Physics. We have over one million books available in our catalogue for you to explore.