
- 232 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
This edition aims to expand on the first edition and take the reader through to the wave equation on coaxial cable and free-space by using Maxwell's equations. The new chapters include time varying signals and fundamentals of Maxwell's equations. This book will introduce and discuss electromagnetic fields in an accessible manner. The author explains electroconductive fields and develops ideas relating to signal propagation and develops Maxwell's equations and applies them to propagation in a planar optical waveguide. The first of the new chapters introduces the idea of a travelling wave by considering the variation of voltage along a coaxial line. This concept will be used in the second new chapter which solves Maxwell's equations in free-space and then applies them to a planar optical waveguide in the third new chapter. As this is an area that most students find difficult, it links back to the earlier chapters to aid understanding. This book is intended for first- and second-year electrical and electronic undergraduates and can also be used for undergraduates in mechanical engineering, computing and physics. The book includes examples and homework problems.
- Introduces and examines electrostatic fields in an accessible manner
- Explains electroconductive fields
- Develops ideas relating to signal propagation
- Examines Maxwell's equations and relates them to propagation in a planar optical waveguide
Martin Sibley recently retired after 33 years of teaching at the University of Huddersfield. He has a PhD from Huddersfield Polytechnic in Preamplifier Design for Optical Receivers. He started his career in academia in 1986 having spent 3 years as a postgraduate student and then 2 years as a British Telecom-funded research fellow. His research work had a strong bias to the practical implementation of research, and he taught electromagnetism and communications at all levels since 1986. Dr. Sibley finished his academic career as a Reader in Communications, School of Computing and Engineering, University of Huddersfield. He has authored five books and published over 80 research papers.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1 Introduction
1.1 Historical Background
1.2 Atomic Structure
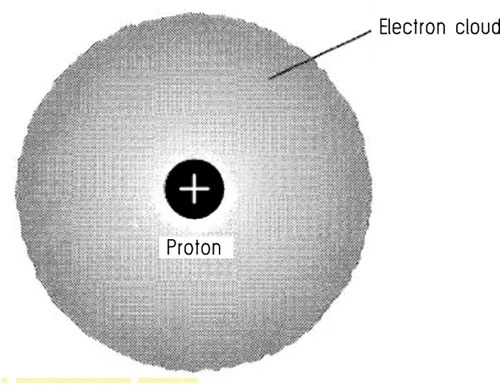
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Dedication
- Table of Contents
- Preface
- Author
- Chapter 1 Introduction
- Chapter 2 Electrostatic Fields
- Chapter 3 Electromagnetic Fields
- Chapter 4 Electroconductive Fields
- Chapter 5 Comparison of Field Equations
- Chapter 6 Dielectrics
- Chapter 7 Ferromagnetic Materials and Components
- Chapter 8 Waves in Transmission Lines
- Chapter 9 Maxwell’s Equations and Electromagnetic Waves
- Chapter 10 The Planar Optical Waveguide
- Problems
- Bibliography
- Index