
- 660 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Fundamentals of Nuclear Science and Engineering
About this book
Fundamentals of Nuclear Science and Engineering, Third Edition, presents the nuclear science concepts needed to understand and quantify the whole range of nuclear phenomena. Noted for its accessible level and approach, the Third Edition of this long-time bestselling textbook provides overviews of nuclear physics, nuclear power, medicine, propulsion, and radiation detection. Its flexible organization allows for use with Nuclear Engineering majors and those in other disciplines. The Third Edition features updated coverage of the newest nuclear reactor designs, fusion reactors, radiation health risks, and expanded discussion of basic reactor physics with added examples. A complete Solutions Manual and figure slides for classroom projection are available for instructors adopting the text.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
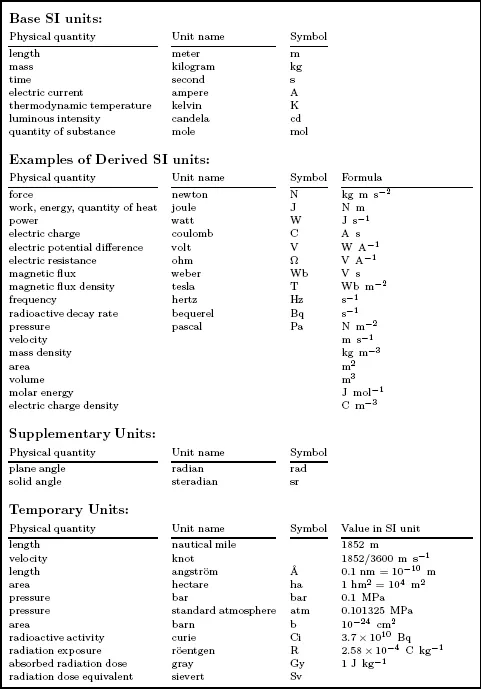
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- 1 Fundamental Concepts
- 2 Modern Physics Concepts
- 3 Atomic/Nuclear Models
- 4 Nuclear Energetics
- 5 Radioactivity
- 6 Binary Nuclear Reactions
- 7 Radiation Interactions with Matter
- 8 Detection and Measurement of Radiation
- 9 Radiation Doses and Hazard Assessment
- 10 Principles of Nuclear Reactors
- 11 Nuclear Power
- 12 Fusion Reactors and Other Conversion Devices
- 13 Nuclear Technology in Industry and Research
- 14 Medical Applications of Nuclear Technology
- A Fundamental Atomic Data
- B Atomic Mass Table
- C Cross Sections and Related Data
- D Decay Characteristics of Selected Radionuclides