Chapter 1: Temperature
The concept of temperature is fundamental to any study of thermodynamics. You have an intuitive sense of temperature, because you can feel if an object is hot or cold. However, like some other fundamental quantities in physics (think for example of time or electric charge), it is not easy to give a precise definition of temperature. To do so, it is necessary to define some other basic concepts and introduce the so-called “zeroth law of thermodynamics,” which leads to the definition of thermal equilibrium; from this, temperature can be defined in an unambiguous way.
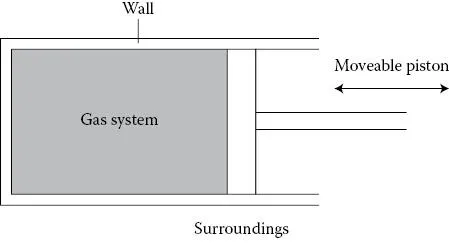
In thermodynamics, attention is focused on a particular part of the universe, simply defined as the system. The rest of the universe outside the system is called the surroundings. The system and the surroundings are separated by a boundary or partition and they may, in general, exchange energy and matter, depending on the nature of the partition. For now consider the exchange of energy only, which makes for a closed system, so that there is no matter exchange between system and surroundings.
A useful example of a system is a fixed mass of compressible fluid, such as a gas, contained in a cylinder with a moveable piston as shown in Figure 1-1. This simple system serves as a model for developing some important ideas in thermodynamics.
First consider a system that is completely isolated from its surroundings. The degree of isolation from external influences can vary over a very wide range, and it is possible to imagine walls that make the isolation complete. In practice, the rigid walls of an ordinary vacuum flask are a good approximation to completely isolating walls. It is an important fact of experience that, after a time, this gas system, or any other system contained in such isolating walls, tends to an equilibrium state in which no further changes occur. In particular, the pressure P becomes uniform throughout the gas and remains constant in time, as does the volume V. We say that the gas is in the equilibrium state (P, V). It is a further fact of experience that, specifying these equilibrium values of the pair of independent variables P and V, together with the mass, fixes all the macroscopic or bulk properties of the gas—for example, the thermal conductivity and the viscosity. A second sample of the same amount of gas with the same equilibrium values for P and V, but not necessarily of the same shape, would have the same viscosity as the first. These ideas can be generalized into the following definition:
An equilibrium state is one in which all the bulk physical properties of the system are uniform throughout the system and do not change with time.
Later you will see that there are other simple thermodynamic systems, apart from a gas, where it is necessary to use other pairs of independent variables to specify the equilibrium state. For a stretched wire system, for example, the appropriate pair is the tension F and length L. Other examples will arise in Section 1.2.3. The important point is that two variables are required to specify the equilibrium state of a simple system. Such directly measurable variables are called state variables. Other common names are thermodynamic variables and thermodynamic coordinates.
The basic state variables P, V, and temperature T are used to define other functions, which take unique values at each equilibrium state. Some examples of such functions are the internal energy, the entropy, and the enthalpy. They are examples of state functions. It is important to realize that it does not matter how a particular state was reached; the value of a state function is always the same for a system in a given state and in no way depends on its past history. State property is an alternative and perhaps a more appropriate name for state function. It will be shown in Section 1.3 that P, V, and T are functionally connected at each equilibrium state by the equation of state, and therefore it is possible to express any one in terms of the other two. Thus these quantities are themselves state functions, but we give them the additional name of state variables because they are easily measured and allow us to specify an equilibrium state in a convenient, practical way.
There are different ways to change the pressure or volume of a gas system. For example, the piston in Figure 1-1 can be pushed in (to the left). This is an example of a mechanical interaction between the system and the surroundings. Pushing the piston in clearly reduces the volume, and in the process the gas’s pressure is likely to change too.
Suppose now that no mechanical interaction is allow...