
- 416 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Enzyme Kinetics and Mechanism
About this book
Enzyme Kinetics and Mechanism is a comprehensive textbook on steady-state enzyme kinetics. Organized according to the experimental process, the text covers kinetic mechanism, relative rates of steps along the reaction pathway, and chemical mechanism—including acid-base chemistry and transition state structure.
Practical examples taken from the literature demonstrate theory throughout. The book also features numerous general experimental protocols and how-to explanations for interpreting kinetic data.
Written in clear, accessible language, the book will enable graduate students well-versed in biochemistry to understand and describe data at the fundamental level. Enzymologists and molecular biologists will find the text a useful reference.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Enzyme Kinetics and Mechanism by Paul F. Cook,W. W. Cleland in PDF and/or ePUB format, as well as other popular books in Medicine & Biochemistry in Medicine. We have over one million books available in our catalogue for you to explore.
Information
1
NOMENCLATURE
An understanding of the language of steady-state kinetics is an important prerequisite to an understanding of the theory and experimental approaches employed by the kineticist. This chapter is devoted to a discussion of the nomenclature developed by Cleland (BBA 67, 104).
Reaction Components
Enzymes catalyze chemical reactions with specific reactants that are called substrates and products. Rather than referring to the actual name of the reactants, a convenient shorthand notation has been developed in which reactants, inhibitors, activators, and enzyme are replaced by letters. Substrates are designated by the letters A, B, C, and D in the order to which they add to enzyme, and products are designated P, Q, R, and S in the order that they are released from the enzyme surface. To illustrate, the alcohol dehydrogenase reaction is shown in equation 1-1, where A and B represent NAD+ and ethanol, and P and Q represent acetaldehyde and NADH:
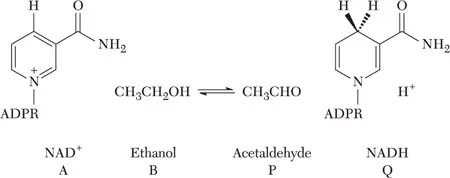 | (1-1) |
A metal ion activator can be designated by M and the ionic charge or by the actual chemical symbol for the metal ion, for exampe, M2+ or Mg2+, while other activators are designated by X, Y, and Z. The letters E, F, and G are reserved for enzyme forms as will be discussed further. The product of an enzyme-catalyzed reaction is a substrate in the opposite direction for a reversible reaction. Thus, the physiologic reaction direction (if known) is chosen for substrates, but it is always best to specify the reaction direction. Inhibitors are designated I and J. The following two reactions schematically depict enzyme combining with substrate to generate product and the combination of enzyme and inhibitor, respectively:
(1-2) |
(1-3) |
There are two kinds of enzyme forms called stable and transitory. An enzyme form is termed stable if, when isolated from the rest of the reaction components, it has a long half-life with respect to the assay time scale, and reactants add to it in a bimolecular step. Stable enzyme forms that occur in a single reaction cycle, that is, conversion of one molecule of reactant to product, are given the abbreviation E, F, or G. An enzyme form is termed transitory if, when isolated from the rest of the reaction components, its half-life is short relative to the assay time scale. The latter are usually enzyme–reactant complexes. In equations 1-2 and 1-3, E is a stable enzyme form while EA, EP, and EI are transitory enzyme forms, since they form and decompose rapidly. Consider the examples given in equations 1-4 and 1-5, where E and F are stable enzyme forms and EA, FP, FB, EQ, EAB, and EPQ are transitory enzyme forms. Differences between E and F will be discussed in detail later, but they are chemically and/or structurally different forms of the enzyme.
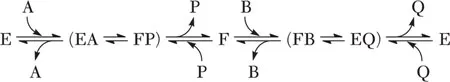 | (1-4) |
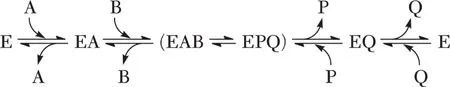 | (1-5) |
There are two types of transitory enzyme complexes, those in which the active site is fully occupied by substrates and products and those in which it is partially filled. Transitory complexes in which the active site is completely filled and kinetically competent are called central complexes, while the others are termed noncentral. The transitory enzyme complexes are denoted by E and the bound reactant, for example, EA and EQ. Central complexes are sometimes additionally enclosed in parentheses, for example, (EAB) and (EPQ), or with both reactants in parentheses along with arrows to show the interconversion of EAB and EPQ. Complexes with one, two, or three reactants and/or products bound are called binary, ternary, and quaternary. In equation 1-4, EA, FP, FB, and EQ are binary central complexes, while in equation 1-5, EAB and EPQ are ternary central complexes. Noncentral complexes are not present in equation 1-4, while EA and EQ are noncentral transitory complexes in equation 1-5.
Kinetic Mechanism
Kinetic mechanisms, that is, the order of addition of reactants to and release of products from the enzyme active site, fall into two classes. Reactions in which all reactants must be bound to an enzyme before any reaction occurs are called sequential, while those in which a product is released between the addition of two substrates are called ping-pong.
A sequential kinetic mechanism is depicted in equation 1-5; both A and B are bound to E before conversion to P and Q takes place. For this type of reaction there is usually only a single stable enzyme form present, E. Sequential mechanisms can be termed either ordered, when there is an obligatory order of addition of reactants to an enzyme and/or release of products from enzyme, or random, when there is not. The term rapid equilibrium is used to indicate a special case in which the equilibrium between an enzyme–reactant complex and free reactant is established in a reaction cycle. The latter will occur when subsequent steps are slow compared to the rate of dissociation of reactant.
The example shown in equation 1-4 depicts a ping-pong kinetic mechanism in which P is released prior to the addition of B. Two stable enzyme forms are present in this type of reaction, and these enzyme forms can usually be isolated. In the ping-pong mechanism a reactant binds to the enzyme active site, undergoes a chemical transformation, and leaves a fragment (or part of the substrate) on the enzyme prior to dissociating as a product. The second reactant then binds, undergoes a second chemical transformation...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Dedication
- Table of Contents
- Preface
- Note to Reader
- Chapter 1 Nomenclature
- Chapter 2 Introduction to Kinetics
- Chapter 3 Enzyme Assays
- Chapter 4 Derivation of Initial Velocity Rate Equations and Data Processing
- Chapter 5 Initial Velocity Studies in the Absence of Added Inhibitors
- Chapter 6 Initial Velocity Studies: Presence of Added Inhibitors
- Chapter 7 Pre-Steady-State and Relaxation Kinetics
- Chapter 8 Isotopic Probes of Kinetic Mechanism
- Chapter 9 Isotope Effects as a Probe of Mechanism
- Chapter 10 pH Dependence of Kinetic Parameters and Isotope Effects
- Appendices
- Index