
- 598 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Fundamentals of Polymer Engineering, Third Edition
About this book
Exploring the chemistry of synthesis, mechanisms of polymerization, reaction engineering of step-growth and chain-growth polymerization, polymer characterization, thermodynamics and structural, mechanical, thermal and transport behavior of polymers as melts, solutions and solids, Fundamentals of Polymer Engineering, Third Edition covers essential concepts and breakthroughs in reactor design and polymer production and processing. It contains modern theories and real-world examples for a clear understanding of polymer function and development. This fully updated edition addresses new materials, applications, processing techniques, and interpretations of data in the field of polymer science. It discusses the conversion of biomass and coal to plastics and fuels, the use of porous polymers and membranes for water purification, and the use of polymeric membranes in fuel cells. Recent developments are brought to light in detail, and there are new sections on the improvement of barrier properties of polymers, constitutive equations for polymer melts, additive manufacturing and polymer recycling.
This textbook is aimed at senior undergraduate students and first year graduate students in polymer engineering and science courses, as well as professional engineers, scientists, and chemists. Examples and problems are included at the end of each chapter for concept reinforcement.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1 | Introduction |
(1.1.1) |
Some Common Polymers
Commodity Thermoplastics | |
Polyethylene | |
Polystyrene | 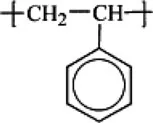 |
Polypropylene | |
Polyvinyl chloride | |
Polymers in electronic applications Polyacetylene | |
Poly(p-phenylene vinylene) | 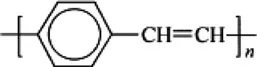 |
Polythiophene | 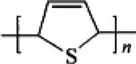 |
Polyphenylene sulfide | 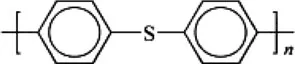 |
Polyanilines | |
Biomedical applications | |
Polycarbonate (diphenyl carbonate) | 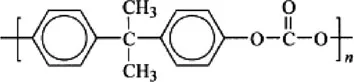 |
Polymethyl methacrylate | |
Silicone polymers | |
Specialty polymers Polyvinylidene chloride | |
Polyindene | 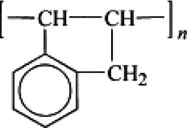 |
Polyvinyl pyrrolidone | 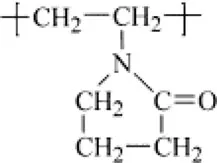 |
Coumarone polymer | 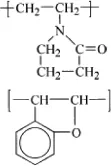 |
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Dedication
- Table of Contents
- Preface to the Third Edition
- Authors
- Chapter 1 Introduction
- Chapter 2 Effect of Chemical Structure on Polymer Properties
- Chapter 3 Step-Growth Polymerization
- Chapter 4 Reaction Engineering of Step-Growth Polymerization
- Chapter 5 Chain-Growth Polymerization
- Chapter 6 Reaction Engineering of Chain-Growth Polymerization
- Chapter 7 Emulsion Polymerization
- Chapter 8 Measurement of Molecular Weight and Its Distribution
- Chapter 9 Thermodynamics of Polymer Mixtures
- Chapter 10 Theory of Rubber Elasticity
- Chapter 11 Polymer Crystallization
- Chapter 12 Mechanical Properties
- Chapter 13 Polymer Diffusion
- Chapter 14 Flow Behavior of Polymeric Fluids
- Chapter 15 Polymer Processing
- Index