
- 401 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Air Quality
About this book
The sixth edition of a bestseller, Air Quality provides students with a comprehensive overview of air quality, the science that continues to provide a better understanding of atmospheric chemistry and its effects on public health and the environment, and the regulatory and technological management practices employed in achieving air quality goals. Maintaining the practical approach that has made previous editions popular, the chapters have been reorganized, new material has been added, less relevant material has been deleted, and new images have been added, particularly those from Earth satellites.
New in the Sixth Edition
- New graphics, images, and an appended list of unit conversions
- New problems and questions
- Presents all-new information on the state of air quality monitoring
- Provides the latest updates on air quality legislation in the United States
- Updates the effects of air pollution and CO2 on climate change
- Examines the effects of the latest changes in energy production and the related emissions and pollutants
- Offers broadened coverage of air pollutant emissions and air quality in a global context
This new edition elucidates the challenges we face in our efforts to protect and enhance the quality of the nation's air. It also highlights the growing global awareness of air quality issues, climate change, and public health concerns in the developing world. The breadth of coverage, review questions at the end of each chapter, extensive glossary, and list of readings place the tools for understanding into your students' hands.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1 Atmosphere
1.1 Chemical Composition
1.1.1 General Composition
| Chemical Species | Symbol | Concentration (%, ppmv, ppbv, pptv) |
|---|---|---|
Nitrogen | N2 | 78.084% |
Oxygen | O2 | 20.948 |
Argon | Ar | 0.934 |
Water vapor | H2O | 0.1–30,000 ppmv |
Carbon dioxide | CO2 | ~412 |
Neon | Ne | 18.18 |
Ozone (stratosphere) | O3 | 0.5–10 |
Helium | He | 5.24 |
Methane | CH4 | ~1.87 |
Krypton | Kr | 1.14 |
Hydrogen | H2 | 0.50 |
Xenon | Xe | 0.09 |
Nitrous oxide | N2O | ~0.33 |
Carbon monoxide | CO | 110 ppbv (50–200) |
Ozone | O3 | 20 |
Ammonia | NH3 | 4 |
Formaldehyde | HCOH | 0.1–1 |
Sulfur dioxide | SO2 | ~1 |
Nitrogen dioxide | NO2 | ~1 |
Carbonyl sulfide | COS | 500 pptv |
Carbon disulfide | CS2 | 1–300 |
Dimethyl sulfide | (CH3)2S | 10–100 |
Hydrogen sulfide | H2S | ~50 |
Nitric oxide | NO | ~50 |
Hydroxyl radical | OH | 0.1–10 |
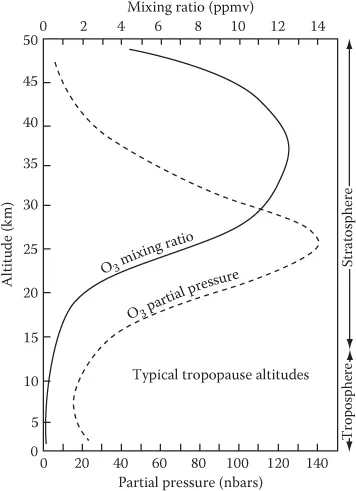
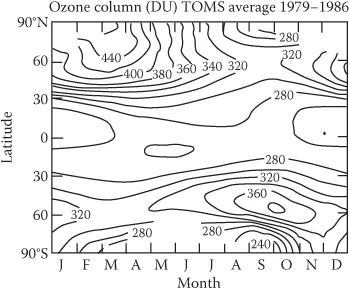
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Dedication
- Preface
- Acknowledgments
- Authors
- Chapter 1 Atmosphere
- Chapter 2 Atmospheric Pollution and Pollutants
- Chapter 3 Atmospheric Dispersion, Transport, and Deposition
- Chapter 4 Atmospheric Effects
- Chapter 5 Health Effects
- Chapter 6 Welfare Effect
- Chapter 7 Air Quality and Emissions Assessment
- Chapter 8 Regulation and Public Policy
- Chapter 9 Control of Motor Vehicle Emissions
- Chapter 10 Control of Emissions from Stationary Sources
- Chapter 11 Indoor Air Quality
- Chapter 12 Environmental Noise
- Glossary
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app