- English
- ePUB (mobile friendly)
- Available on iOS & Android
CO2 Hydrogenation Catalysis
About this book
A guide to the effective catalysts and latest advances in CO2 conversion in chemicals and fuels
Carbon dioxide hydrogenation is one of the most promising and economic techniques to utilize CO2 emissions to produce value-added chemicals. With contributions from an international team of experts on the topic, CO2 Hydrogenation Catalysis offers a comprehensive review of the most recent developments in the catalytic hydrogenation of carbon dioxide to formic acid/formate, methanol, methane, and C2+ products.
The book explores the electroreduction of carbon dioxide and contains an overview on hydrogen production from formic acid and methanol. With a practical review of the advances and challenges in future CO2 hydrogenation research, the book provides an important guide for researchers in academia and industry working in the field of catalysis, organometallic chemistry, green and sustainable chemistry, as well as energy conversion and storage. This important book:
- Offers a unique review of effective catalysts and the latest advances in CO2 conversion
- Explores how to utilize CO2 emissions to produce value-added chemicals and fuels such as methanol, olefins, gasoline, aromatics
- Includes the latest research in homogeneous and heterogeneous catalysis as well as electrocatalysis
- Highlights advances and challenges for future investigation
Written for chemists, catalytic chemists, electrochemists, chemists in industry, and chemical engineers, CO2 Hydrogenation Catalysis offers a comprehensive resource to understanding how CO2 emissions can create value-added chemicals.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Introduction
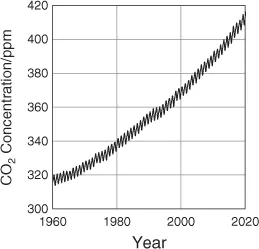
1.1 Direct Use of CO2
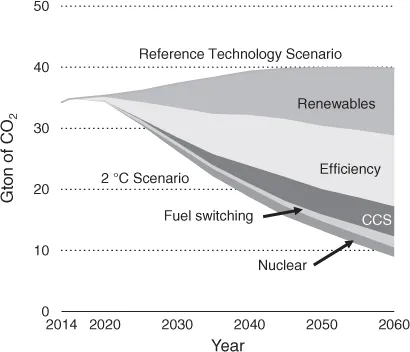
1.2 Chemicals from CO2 as a Feedstock
Table of contents
- Cover
- Table of Contents
- CO2 Hydrogenation Catalysis
- Copyright
- Preface
- 1 Introduction
- 2 Homogeneously Catalyzed CO2 Hydrogenation to Formic Acid/Formate by Using Precious Metal Catalysts
- 3 Homogeneously Catalyzed CO2 Hydrogenation to Formic Acid/Formate with Non‐precious Metal Catalysts
- 4 Catalytic Homogeneous Hydrogenation of CO2 to Methanol
- 5 Theoretical Studies of Homogeneously Catalytic Hydrogenation of Carbon Dioxide and Bioinspired Computational Design of Base‐Metal Catalysts
- 6 Heterogenized Catalyst for the Hydrogenation of CO2 to Formic Acid or Its Derivatives
- 7 Design and Architecture of Nanostructured Heterogeneous Catalysts for CO2 Hydrogenation to Formic Acid/Formate
- 8 Heterogeneously Catalyzed CO2 Hydrogenation to Alcohols
- 9 Homogeneous Electrocatalytic CO2 Hydrogenation
- 10 Recent Advances in Homogeneous Catalysts for Hydrogen Production from Formic Acid and Methanol
- Index
- End User License Agreement
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app