![]()
PART ONE
NATURAL PROCESSES
In part 1 of this book, I describe processes in biological systems, such as bacteria, plants or animals, but only insofar as this is relevant to the future of our world. It is useful to know about these natural processes for two reasons. First, we should know these physical, chemical, and biological processes because we as humans depend on biological organisms for our food. In our future society, matters may be arranged similarly: mimicking green plants, some chemical procedures will split water into hydrogen and oxygen with the help of solar energy. We will then burn the hydrogen again; that is, we will bind it again to the oxygen released earlier. Thereby, we will obtain the energy to go for a jog; run our machines, equipment, and cars; make new chemical compounds; and drive our programs to recycle the waste we unavoidably make. In this way, the study of natural processes and their origin teaches us how we can persist on Earth in a complex human society. And, second, it is important to know how, in their evolution, organisms have become organized internally and in relation to each other by continually adapting to stressful situations. This organization may suggest how we should organize human society in future too. We can learn by example.
1 THE NATURE OF LIFE: MAKING WASTE
Feeding adds matter and energy to an organism. Yet, after some time, an organism gets hungry and begins to feed again. Having to feed again means that energy and matter have been used somehow; they are used to sustain the organism’s life, and the byproduct is waste expelled from the organism.
In very broad terms, this is what happens to all organisms all the time. Organisms big and small, simple and complex. Plants and animals, bacteria, and molds. Life is a never-ending stream of energy and matter—a stream entering a system as energy-rich food, doing work inside, and leaving it again as energy-poor waste. Actually, this stream has already flowed uninterruptedly for almost four billion years. Before they die and hand over the flame to their off spring, all organisms feed and turn food into waste, which they expel. This continues for generation after generation of organisms. Their waste, and eventually their bodies, become food for yet other organisms. And so on, and so on. Indefinitely. This food can be water or minerals from the soil, plants or animals, or gases from the air. The same perpetual cycle applies to waste: the water vapor we breathe out, the minerals returning to the soil, the oxygen plants release into water or air—or the dead elephant decaying away, over the months returning to the soil and air all its constituents assembled and used over the years. Except, of course, its energy, which it has continually dissipated throughout its life, first into the environment, and from there eventually into outer space—from which it originally reached us as solar energy.
In one way or another, the result is a never-ending stream of energy and matter flowing through several kinds of organism: from food to plant to animal to waste. Put crudely, an organism is an organized flow of energy and matter, ending up as waste. Or, more succinctly, it is a mechanism processing resources into waste. The energy and matter together form this mechanism, which both transfers and stores them for some time. This extremely intricate mechanism is what we know as an organism. And together, many organisms using each other’s waste as their resource, as food, form a mechanism recycling mineral resources. These cycles are driven by energy that at present comes from the sun.
In slightly more detail, what happens is more complicated: the food has often first been stored within the organism and is used to power various life functions. Apart from storing energy and matter, organisms also grow, becoming larger and larger. Obviously, energy and matter are needed just to enable an organism to become larger (that is, an organism needs food so it can grow). Energy and matter are also used to maintain the organism. Like most things, all organisms wear out and decay; unavoidably, something goes wrong in the very complex and intricate processes within the organism’s body so that its parts must constantly be repaired if they are to maintain their original function. These ongoing repairs are powered by the energy and matter in the organism’s food. This maintenance is usually insufficient to restore all functions adequately, however, and ultimately the organism dies.
Yet another way matter and energy are used is for reproduction. Parents reproduce themselves in new, young organisms—their off spring; these new organisms eventually take the place of their parents when the latter wear out and die. Finally, some types of organisms, most of them animals (but never plants and molds), are mobile. This means that to feed or reproduce, for example, they can move from one place to another, using some of the energy which they have ingested.
Having lost energy and material in these various ways, the organism has to replace them. That is why animals get hungry and begin to search for food. Plants also get hungry but they use their food mainly for storage, maintenance, and growth, and at some point in their life cycle also for reproduction, that is, for making flowers and seeds. Plants also get thirsty: they evaporate water with their leaves, and also use part of it for building up their body. They drink water from the soil using their roots. The difference between plants and animals is that it is customary that we never talk about hunger or thirst, eating or drinking of plants, which we do in connection with animals. But in fact, they are doing the same.
Although for some time the energy and matter form part of the organism for all these functions, on its death the organism itself becomes waste, forming food for other organisms, such as bacteria or fungi, which break it down. Thus, the dead elephant mentioned earlier is first used as food by vultures and hyenas, and later what little they leave is consumed by bacteria and fungi. Eventually, all these organisms expel their waste into the environment, where it becomes food for plants again, and so on in an endless cycle of resource use and waste production. The cycle is like a snake biting its own tail, forming a circle. But if the snake didn’t bite its tail and was instead a more or less straight line, the system would be exhaustive: the waste would remain unused forever, never to become food again. This straight line represents the process of resource depletion, and, at the same time, the piling up of waste, which pollutes the environment. By contrast, the two processes of resource depletion and waste production are connected to each other and are consequences of each other. The one does not go without the other. They are yin and yang. Resources enter the processing system and leave it as waste, which turns into food. Without recycling, resources deplete and waste accumulates.
A different pattern holds for the energy use of plants. They derive their energy directly from sunlight. Using this energy, a plant can build up complex chemical compounds from gases in the air and from certain minerals and water in the soil. The plant uses these compounds to build the cells of its body. Thus, the plant’s food consists of solar energy, plus chemical compounds and water from the air and soil. All our energy ultimately comes from the sun and returns back to space. It keeps the great cycles of life processes turning, although overall it is a noncyclic, linear process of energy degradation. By depleting our resources and leaving our waste unused, we similarly follow a noncyclic, linear, exhaustive type of process, not only of energy but also of materials.
The question is, of course, why this difference between linear and cyclic processes? Why are cyclic processes typical for biological systems? Is there a reason nature follows that particular strategy in all organisms, and at all levels, from within organisms (the chemical level) to between organisms (the ecological level)? And why do we apply the simpler, linear process? Is it simply because we don’t adjust the amounts of the production of resources and waste to each other? If anything, one might have expected the opposite to be the case: our sophisticated human society, with all its deliberate planning, might have developed the more complex cyclic type of processes. Homo sapiens is the only knowing species. With our greater intelligence, we would easily be able to master the complexity of cyclic processes, individually and with all their interactions. This would have been impossible for organisms of all those other species that are much less sophisticated than we are. Surely we are cleverer than a bacterium, aren’t we?
But, disturbingly, with regard to recycling, the reverse is true. So, why? You may even wonder why those more complex cyclic processes occur in nature anyway. And, again, are they bound to biological systems and do they happen under all conditions, or are they only found under particular, restrictive conditions under which those systems are found? Moreover, if, one day we found ourselves living under similar, restrictive conditions, would we then need to develop similar systems of cyclic processing—for example, when either resource depletion or waste production become pressing, or perhaps both become a threat to our sustainability, our existence on Earth? How rigorous and rigid ought these cyclic systems be in order to guarantee our sustainability as a species and a society? Also, would they impose restrictions on our behavior, on our resource use, or even on the number of humans occurring on Earth? Would those restrictions be more or less stringent than those found in the nonhuman living world?
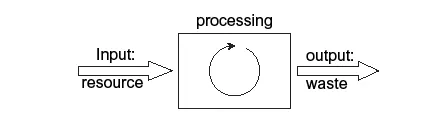
Let’s depict the two types of processes in the form of a diagram. The figure above shows the stretched-out (linear) exhaustive system. It consists of a processing box with an input arrow on the left and an output arrow on the right. The input arrow can represent anything: food, gasoline, heat, garbage, wood chips—whatever. Similarly, the processing box can also represent anything: you yourself digesting your food; your automobile burning the gasoline to make heat, which moves a piston in the engine and ultimately makes the automobile move; and so on. The output at the right can be a product or mere waste. Thus, the product you made can also be something you really wanted to make one day, like a chair or a nicely polished gemstone for your loved one. Or it could be a task you wanted to do, like pollarding a willow tree in a meadow after the winter cold. Or it can be combustion heat—energy—for driving your heavy removal truck uphill, or it can be some wanted chemical product, like sunscreen. Similarly, however, waste as a processing output can also consist of some poison polluting the groundwater or heat lost somewhere in the environment (warming up a nearby river, for example).
Obviously, the amount of matter found on either side of the box must be the same, because we can neither make nor destroy matter. The same amount of matter that comes in at the left of figure, comes out at the right. In fact, the same applies to energy, as we cannot destroy energy either. But there is a difference: we lose unusable energy; it becomes useless heat. As a result of the processing activities inside the box, energy is said to degrade. Plants input solar energy into their chemical system, storing it temporarily, but in the end, they lose it as degraded, unusable heat. And when they are eaten by some animal, it obtains the stored energy, which it decomposes, releasing the energy as heat. That is no recycling either—it is extending the linear process the plants followed. And the same happens when we eat plant or animal food or when we burn oil, natural gas, or coal as fossil remains of ancient algae and plants. So, in all systems, there is an overall loss of energy in the form of heat. We degrade energy to a lower form by using it, but it is still there at the end of the process, and in the same amount—but in a useless form. We can release energy by burning wood or oil in stoves or in turbines or by digesting starch in the cells of our bodies. Or we can extract it from the heat radiated by the sun. Therefore, similar to matter, neither can we make energy ourselves, nor can we destroy it. And here’s the difference between energy and matter: we can recycle matter, but we cannot recycle energy. If we want to recycle energy, we would first have to upgrade it again from its degraded form, which is impossible. Matter can be recycled, whereas energy always follows this linear, down-slope path of resource depletion toward waste production. Moreover, the compounds to the right of the box are overall more stable than the compounds to the left. And this has something to do with the overall loss of heat, which in turn has to do with the need to add new energy in order to be able to recycle these stable compounds.
The most general law in physics tells us just this: wherever you look in the universe—at large galactic processes or at small ones within or among atoms—there is always an increase in the amount of waste energy, heat accompanied by a rundown of material organization into disorderly waste. The running of organizations, be they individual organisms, sports clubs, governmental organizations, or societies and civilizations, indicates that energy degrades during this process, and at some point leaves as heat. We can’t escape this law; we can only hope to slow down our own processes of waste production by being economical. And that’s exactly what cyclic systems do. They minimize the amount of material used by recycling it internally. And when some material happens to be thrown away as waste, out of the cell, or out of the body as feces, some of it can still be taken up and used by some other organism—that’s interorganism recycling. This is exactly what has been going on in nature since life began. And it goes on because the resources on Earth are finite, so recycling is imperative.
Energy too is limited. Living systems must have run out of usable chemical energy quite soon after they came into existence. Degraded energy cannot be upgraded again, and waste matter cannot be taken back into use without inputting the energy that was lost when orderly matter became disorderly waste matter. Fortunately, there is an external source of energy available to life on Earth: the energy from our nearest star, the sun. Every day for billions of years, the sun has supplied us with huge amounts of new energy that can be used to convert waste into a reusable form and allow new elements and new processes to be incorporated into new chemical “machinery” added to the existing machinery but using more energy.
So, we—along with plants, animals, bacteria, and fungi—are all driven by solar energy. But where does the sun itself get its energy? Some sixty years ago, we learned that in the core of the sun, the same fusion processes as in the operation of the hydrogen bomb have been going on for billions of years; the sun, therefore, can be considered one enormously large hydrogen bomb. It’s a fusion bomb because two atoms of the hydrogen it contains fuse into one larger chemical element, helium, and this, in turn, fuses into yet other, even larger elements. In a sense, the whole universe appears to contain an unbelievably large number of gigantic hydrogen bombs: the stars. Some of them explode with incredible force, whereas others simply fade away when their hydrogen runs out. Some of the vast amount of energy produced by our sun reaches us here on Earth as light and heat. It is the process of hydrogen fusing into helium that has warmed Earth for all 4.5 billion years of its existence. And the sun will continue to warm Earth for about the same length of time in the future.
It seems that this kind of process occurs throughout the known universe: at first, there was only hydrogen, the element with which all other processes in the universe began. The history of the present state of the universe is written in terms of hydrogen. All chemical elements on Earth—those that make up our bodies and all things and organisms around us—form only a tiny fraction of all the hydrogen that exists. They are completely overshadowed by the enormous amount of hydrogen surrounding us in the universe. On this extremely large scale—as large as the distance covered by light during some fifteen billion years—it’s almost all hydrogen around us, for as far you can see. The solar system, Earth, and we ourselves within it are the great exception.
And hydrogen also proves to be crucial for life. Before life began on Earth, there must have been plenty of hydrogen molecules available in the environment. Life began using these molecules because of the relative ease with which hydrogen can form and break chemical bonds. My personal theory is that most of the available hydrogen was bound first by selenium, and then by sulfur and phosphorus—and of course, still later by oxygen. Hydrogen and oxygen together form the compound water (H2O), which literally fills up whole oceans. Other elements, those able to bind with hydrogen and also able to break up chemical bonds quite easily again, began to cycle hydrogen around and around. However, over time, they unavoidably bound more and more of the free hydrogen molecules. The result was that in order to obtain or release hydrogen from the first elements, other elements that could pull harder on hydrogen and retain it for longer now entered the processes of life. Eventually, carbon was thus included in life processes, in its turn taking a share of the available hydrogen. At the same time, the reaction products of these hard-pulling elements also became less likely to break up; the elements that joined in later pull so hard that they are less reactive, and therefore their molecules are more stable. So, the flip side of the evolutionary coin is that compounds made by recent living organisms are less reactive than they must have been in the most ancient life forms. But retaining the hydrogen longer also meant storing some binding energy longer; in particular, it was the carbon compounds that specialized in energy storage (think of starch in plants or of animal fats and oils).
Yet this meant that plants and animals would also produce nonrecyclable biological waste, that is, nonreactive stable compounds that other organisms could not break down. Also, the organisms became more complex, each of them using a greater number of the available elements. They tended to keep using the former elements in the old mechanisms, which were always kept operating, and they used new elements for their newly patched-up mechanisms. And the complexity grew ever-more rapidly, because any new mechanism had to be supported itself by one or more other ones, and these, in turn, by yet other mechanisms. The more complex forms were larger than the simpler ones and therefore contained more of the available hydrogen. Easily available hydrogen was running out.
Obviously, therefore, the scarcer free hydrogen became, the stronger the elements had to pull to get their share. The initial elements could not start by pulling hard themselves, because their reaction products would be too stable, but they could be substituted by other ones with more pulling capacity. This is why selenium will have been substituted by sulfur, which then formed further compounds with the help of phosphorus. Sulfur may have been the first element to be substituted for selenium, because their chemical properties are similar; it was particularly because of its stronger pulling force that it soon...