
eBook - ePub
Essentials of Pediatric Urology
- 416 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Essentials of Pediatric Urology
About this book
Essentials of Pediatric Urology provides surgical trainees with an up to date and comprehensive account of the urological disorders of childhood . In addition, this popular textbook makes a valuable practical contribution to clinical decision making by Adult Urologists and General Pediatric Surgeons who treat conditions of the genitourinary systems in children. This established resource fulfils a unique role as the only international textbook of Pediatric Urology written primarily for trainees and those practising adult Urology, Pediatric Surgery, and Pediatric Urology. The third edition continues to meet this need as well as providing a ready source of reference for non-specialists including Pediatricians and Nurses.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Essentials of Pediatric Urology by Duncan T Wilcox, David F M Thomas, Duncan T Wilcox,David F M Thomas in PDF and/or ePUB format, as well as other popular books in Medicine & Clinical Medicine. We have over one million books available in our catalogue for you to explore.
Information
1 Embryology
Topics covered
- Genetic basis of genitourinary malformations
- Embryogenesis
- Upper urinary tract
- Lower urinary tract
- Genital tracts
INTRODUCTION
This chapter will concentrate predominantly on the clinical aspects of the embryological development of the genitourinary tract but will also refer to relevant advances in scientific methodology.
Techniques such as polymerase chain reaction (PCR), fluorescence in situ hybridisation (FISH) and studies using transgenic “knockout” mice have greatly advanced our understanding of the genes involved in regulating normal embryological development. In addition, innovations in microscopy and three-dimensional (3D) reconstruction are shedding new light on structural development of the human embryo and fetus.
GAMETOGENESIS, FERTILISATION AND THE GENETIC BASIS OF UROLOGICAL ABNORMALITIES
In males, the formation of gametes (spermatozoa) does not commence until puberty whereas in females it commences during fetal life but does not progress beyond prophase of the first meiotic division. At this stage the primary oocytes enter a dormant phase until the onset of puberty when a small number of primary oocytes resume the long – arrested meiotic division. Under the influence of follicle-stimulating hormone and luteinising hormone, one or two secondary oocytes are extruded from the ovaries at the time of ovulation. Penetration of the protective zona pellucida of the oocyte by a spermatozoon triggers a second meiotic division to create a “definitive oocyte”.
As a result of the meiotic divisions during gametogenesis, the nuclei of the definitive oocyte and spermatozoon contain a single copy of each of the 22 autosomes and 1 sex chromosome. Fusion of the nuclear DNA of the two gametes during fertilisation creates a zygote whose nucleus contains 46 chromosomes – with one copy of each pair of autosomes and one of the two sex chromosome derived from each parent. On its journey along fallopian tube, the newly fertilised zygote undergoes a series of mitotic divisions (termed cleavage) to form a mass of cells termed the blastocyst (Figure 1.1).
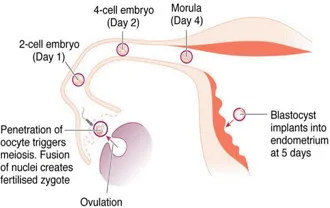
Figure 1.1Key stages between fertilisation and implantation of the blastocyst at 5–6 days.
Major chromosomal abnormalities can arise either during gametogenesis or fertilisation or the early mitotic divisions of the zygote. Most chromosomal abnormalities of this severity lead to spontaneous abortion of the embryo but trisomy 21 (Down syndrome), trisomy 13 (Patau syndrome) and trisomy 18 (Edward syndrome) are compatible with survival. Of these, however, only trisomy 21 is compatible with longer term survival into adult life.
Trisomies can occur as a result of non-disjunction (in which a pair of chromosomes fail to separate during gametogenesis) or translocation (in which a chromosome, or piece of a chromosome, becomes attached to another chromosome during meiotic division).
The corollary of non-disjunction and translocation is the formation of a gamete which lacks one copy of that particular chromosome. This results in the formation of a zygote whose nucleus contains only a single (unpaired) copy of the particular chromosome. This is termed monosomy. Absence of an entire autosome (complete monosomy) invariably leads to spontaneous abortion of the embryo whereas some partial monosomic states are compatible with survival. By contrast to the abnormalities affecting autosomes, major structural abnormalities of the sex chromosomes are not only consistent with survival but are relatively common. Examples include Klinefelter syndrome (47XXY) and Turner syndrome. Approximately 50% cases of Turner syndrome exist as complete monosomy (45X) whilst 30% of cases occur in mosaic form 45X/46XX) and 20% result from a structural deletion of genetic material on one of the X chromosomes. 45X/46XY mosaicism is known as mixed gonadal dysgenesis. Mosaicism is defined as the presence of two genetically distinct cell lines derived from the same zygote. Abnormalities of the sex chromosomes often occur in mosaic form.
Genetic mutations occurring at the level of individual genes can be studied using techniques such as polymerase chain reaction (PCR) and fluorescence in situ hybridisation (FISH). A number of inherited conditions affecting the genitourinary tract can be ascribed to identifiable mutations, e.g. autosomal recessive polycystic kidney disease (ARPKD), autosomal dominant polycystic kidney disease (ADPKD), X-linked Kallmann's syndrome and renal coloboma syndrome. However, attempts to identify specific mutations in common urological conditions with a strong familial tendency such as vesico ureteric reflux, upper tract duplication and hypospadias have been unrewarding. The occurrence of these disorders in members of the same family is more likely to result from the interaction of multiple genes than the effect of a single gene mutation. The possible role of environmental factors in modifying gene expression during embryological development of the genitourinary tract is poorly understood.
EMBRYOGENESIS
Human gestation spans a period of 38 weeks, from fertilisation to birth. The formation of organs and systems occurs mainly between the third and tenth weeks with subsequent development being characterised mainly by differentiation, branching, maturation and growth. By the time the blastocyst implants into the primed endometrium (approximately 6 days after fertilisation) it has undergone organisation to form an outer trophoblastic layer and inner cell mass. Over the ensuing 10 days, the amniotic cavity and the yolk sac develop within the blastocyst – with the embryonic disc forming in the interface between them. Ectodermal tissue originates from cells on the amniotic surface of the embryonic disc whereas endodermal tissues are derived from cells adjacent to the yolk sac. Inpouring of cells into the embryonic disc from the amniotic surface via the primitive streak creates a third layer – the intraembryonic mesoderm (Figure 1.2). It is from the intraembryonic mesoderm that much of the genitourinary tract is ultimately derived.
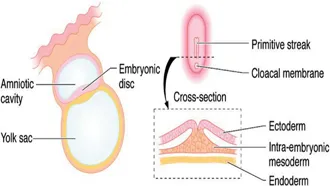
Figure 1.2Embryonic disc at 16 days with inpouring of cells into the primitive streak to create intraembryonic mesoderm.
UPPER URINARY TRACT (Figure 1.3)
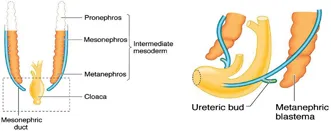
Figure 1.3Precursors of the upper urinary tract, metanephros and ureteric bud.
By the fourth week, two blocks of mesoderm have appeared on each side of the midline. Sequential differentiation within this mesoderm gives rise to the pronephros in the most cephalad region, the mesonephros in the mid-zone and the metanephros in the most caudal region. The pronephros regresses rapidly and serves no function in the human embryo. At around the same time, condensations of mesenchyme lying lateral to the mesonephros undergo canalisation to form the mesonephric ducts, which advance in a caudal direction to merge with the cloaca. Tubular structures within the mesonephros establish a communication with the mesonephric duct to fulfil a transient excretory role until around 10 weeks. These tubules then regress in the female but in the male they persist as precursors of the efferent tubules of the testis.
At around 28 days, the ureteric bud develops as a protrusion from the mesonephric duct. The ureteric bud then advances towards the metanephros to penetrate the metanephric mesenchyme at around 32 days. The formation of nephrons by interaction between the ureteric bud and metanephros occurs by a process of reciprocal interaction between the two tissues – a phenomenon which occurs in the embryological development of a number of systems. Sequential budding and branching of the ureteric bud gives rise to the renal pelvis, the major calices, the minor calices and the collecting ducts whilst the glomeruli, convoluted tubules and loop of Henle are derived from the metanephric mesenchyme (Figure 1.4). The cortex and medulla are discernible by 15 weeks and new generations of nephrons continue to be added to the cortex up until 36 weeks. In humans, the process of nephrogenesis ceases at 36 weeks and the total number of nephrons remains fixed thereafter at approximately 1 million per kidney. Nephron numbers are reduced in the kidneys of preterm and low-birth-weight infants. Almost 3000 different genes have been identified as being involved in ureteric bud formation and nephrogenesis. The roles played by many of these genes have been studied in transgenic mice and in clinical genetic studies. Of these, the gene encoding for glial cell line-derived neurotophic factor (GDNF), Wilms tumour suppressor gene (WT1) and RET proto-oncogene have been shown to play key roles.
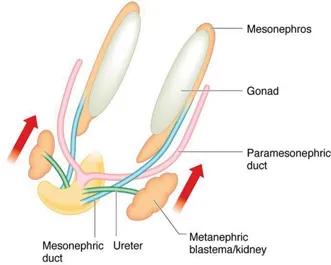
Figure 1.4Embryonic urinary tract at 6–8 weeks.
Renal Dysplasia
Although “dysplastic” is often used loosely to refer to any congenitally small kidney, the term “dysplasia” refers more accurately to kidneys demonstrating certain characteristic histological features. These include disordered renal architecture, immature “primitive” undifferentiated tubules, small cysts and the inapp...
Table of contents
- Cover Page
- Half Title Page
- Title Page
- Copyright Page
- Dedication Page
- Contents
- Contributors
- Preface
- Acknowledgements
- 1 Embryology
- 2 Renal Development and Dysfunction
- 3 Imaging
- 4 Prenatal Diagnosis
- 5 Urinary Tract Infection
- 6 Vesicoureteral Reflux
- 7 Upper Tract Obstruction
- 8 Duplication Anomalies, Ureteroceles and Ectopic Ureters
- 9 Posterior Urethral Valves and Other Urethral Abnormalities
- 10 Cystic Renal Disease
- 11 Urinary Tract Calculi
- 12 Urinary Incontinence
- 13 Neurogenic Bladder
- 14 Urologic Anomalies in Anorectal Malformations and Renal Ectopia
- 15 Bladder Exstrophy and Epispadias
- 16 Hypospadias
- 17 The Prepuce
- 18 Testis, Hydrocoele and Varicocoele
- 19 The Acute Scrotum
- 20 Disorders of Sex Development
- 21 Genitourinary Malignancies
- 22 Pediatric Genitourinary Trauma
- 23 Laparoscopic Paediatric Urology
- 24 Adolescent Urology
- 25 Pediatric and Adolescent Gynecology
- Appendix I Self-Assessment Section: Questions
- Appendix II Self-Assessment Section: Answers
- Index