
eBook - ePub
Rehabilitation of Visual Disorders After Brain Injury
Josef Zihl
This is a test
- 200 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Rehabilitation of Visual Disorders After Brain Injury
Josef Zihl
Book details
Book preview
Table of contents
Citations
About This Book
Despite a long research tradition in visual neuroscience, the rehabilitation of cerebral visual deficits has, until recently, been neglected. This book is the first to report systematic observations on spontaneous recovery of cerebral visual deficits after acquired brain injury, and the outcome of treating these deficits. The whole range of human visual functions and capacities is covered: visual field, visual acuity and contrast sensitivity, visual adaptation, colour vision, visual space perception, and visual cognition. Additionally, there is a special section devoted to patients with central scotoma. All treatment procedures described are empirically founded.
Frequently asked questions
How do I cancel my subscription?
Can/how do I download books?
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
What is the difference between the pricing plans?
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
What is Perlego?
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Do you support text-to-speech?
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Is Rehabilitation of Visual Disorders After Brain Injury an online PDF/ePUB?
Yes, you can access Rehabilitation of Visual Disorders After Brain Injury by Josef Zihl in PDF and/or ePUB format, as well as other popular books in Medizin & Physiotherapie, Physikalische & Rehabilitative Medizin. We have over one million books available in our catalogue for you to explore.
Information
CHAPTER ONE
Introduction
From the very beginning of neuroscience, vision research has mainly been concerned with the elucidation of the nature of various visual deficits and the identification of the location of brain injury responsible for these deficits (for a comprehensive review, see Grüsser & Landis, 1991). Early clinical reports on patients showing selective visual loss following posterior brain injury have suggested that visual functions are cortically distributed, a concept that many years later has been verified on the basis of combined anatomical, electrophysiological, and behavioural evidence (Desimone & Ungerleider, 1989; Felleman & van Essen, 1991; Zeki, 1978, 1993). Enormous progress has been made in understanding the neurobiological basis of visual perception, and it is meanwhile generally accepted that the visual cortex is functionally specialised and builds up flexible networks to subserve complex visual abilities, such as recognising objects or orienting in space (Corbetta, Miezin, Shulman, & Petersen, 1993; Tootell, Dale, Sereno, & Malach, 1996). The neuropsychology of vision is still a major topic in neuroscience, and the questions of how the different lower and higher level “processing units” integrate pieces of information, how they co-operate by various interactions to achieve and maintain coherence of visual perception in time and space, and how they are influenced by attention and intention etc., are exciting and very promising research topics for the next few years (see Cowey, 1994, and Driver & Mattingley, 1995, for reviews).
In contrast to the numerous contributions to the understanding of cerebral organisation of visual perception, few, however, are devoted to the study of recovery of visual function in patients with acquired brain injury. This, at first sight, is difficult to understand given the fact that, for example, between 20% and 40% of patients with stroke suffer from visual disorders (Hier, Mondlock, & Caplan, 1983a; Sarno & Sarno, 1979). Furthermore, visual disorders may affect cognitive performance (Uzzell, Dolinskas, & Langfitt, 1988) and may reduce the effect of rehabilitation measures, thereby impairing vocational rehabilitation (Groswasser, Cohen, & Blankstein, 1990; Reding & Potes, 1988). The reason for this is certainly not the missing interest in the recovery of vision and visual rehabilitation, and furthermore cannot be due to a lack of acknowledgement of this field of brain research. As early as 1867, Zagorski reported the case of a 35-year-old lady who complained of loss of vision on the left side. Perimetric testing revealed a complete left-sided hemianopia, probably caused by a right-sided occipital haemorrhage. Eight days later the patient noticed return of light vision in the left hemifield; 6 weeks later she reported to have full vision again. Perimetric testing of visual fields was performed in intervals of about 1 week. The results were in agreement with the report given by the patient: The region of blindness shrank successively and vision eventually returned to the left hemifield (see Fig. 1.1). This is probably the first report on recovery of vision after brain injury. In their Handbook for neurologists and ophthalmologists Wilbrand and Saenger (1917) dedicated a comprehensive chapter to the incidence and course of homonymous hemianopia. According to their observations, in patients with complete cortical blindness, vision recovered first in one hemifield; a few cases later showed complete return of vision. In most of the cases, recovery of vision took place within hours or days; in some patients, however, the process of recovery was much slower and was not completed for several weeks. A similar course was observed in cases with homonymous hemianopia. In the same year (1917), Poppelreuter (1917/1990) published his monograph on visual disturbances after occipital gunshot wounds, in which he not only reported the results of his detailed testing of visual disorders, but also his observations on spontaneous recovery and on the effect of systematic treatment. Poppelreuter’s approach was experimentally oriented and at the same time was a very pragmatic one. This is exemplified by his statement (p. 5) that “any intervention should, at the very least, have as its aim that the man should again be able to converse comprehensibly, to write his own letters, to read a newspaper, and to calculate his expenses by himself”. Poppelreuter pointed out that functional impairments in the acute stage may often be exaggerated. However, complete spontaneous recovery was the exception rather than the rule; rehabilitation measures were therefore required in most of his patients. Poppelreuter was aware of the difficulty of attributing an improvement unequivocally to the treatment (p. 240): “Only exact control of the effect [of treatment] offers a substantial argument for the systematic training effect over a short period of time, namely using a work task which remains constant.” He developed training methods especially aimed at improving reading in patients with visual field loss. He had noticed that parafoveal field loss not only impaired the perception of words but also the guidance of reading eye movements. Therefore he taught patients to compensate for their field loss by systematically shifting fixation from the beginning to the end of a line. The resulting improvement in reading is the first known example for the substitution of cerebral visual loss by oculomotor activities. According to Poppelreuter, the substitution of one function (the parafoveal visual field) by another (eye movement) is crucially dependent on whether, under normal circumstances, the substituting function also contributes to the performance in question.
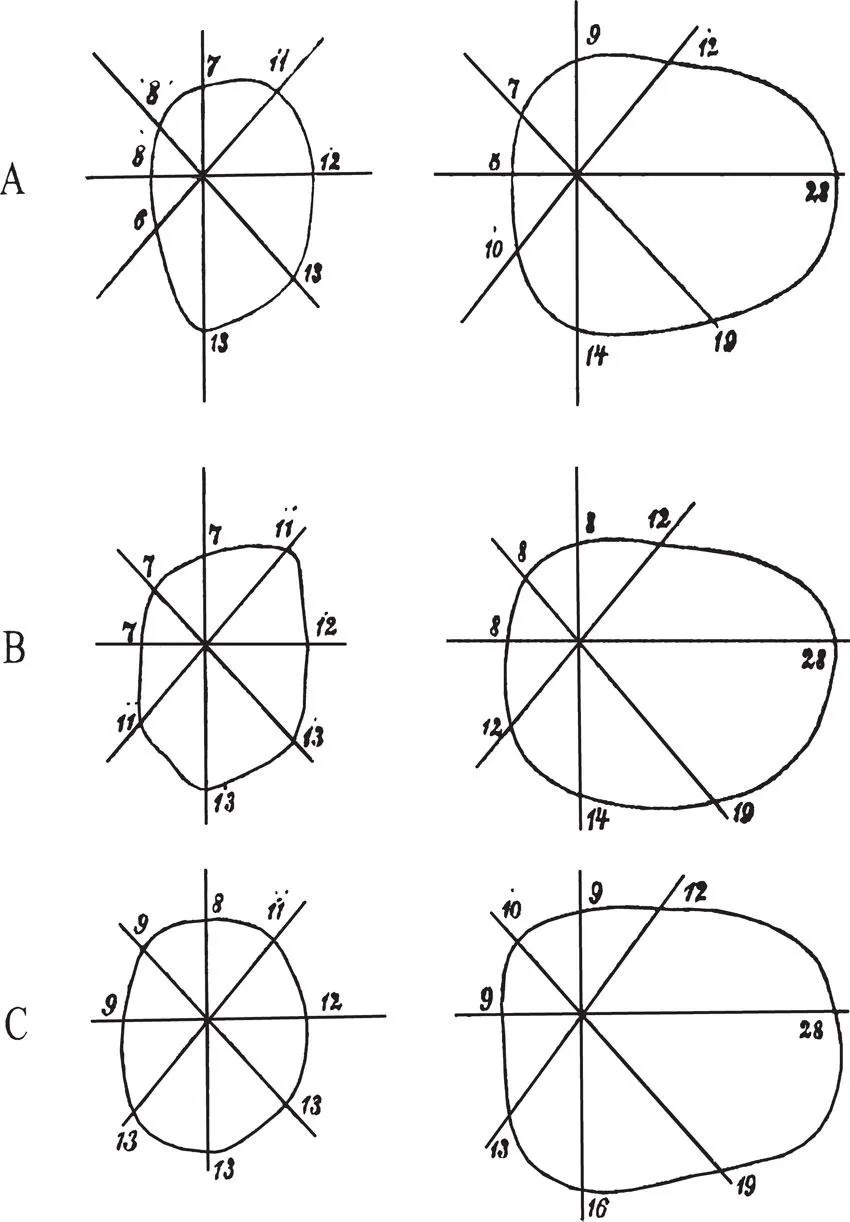
Poppelreuter’s observations on the recovery of vision after brain injury and his experiences with the systematic treatment of visually disabled, brain-injured patients were for the most part neglected in the literature, as was the aspect of recovery of visual function in patients with occipital injury. Even in the classic monograph by Teuber, Battersby, and Bender (1960) on visual disturbances in a similar group of cases, namely World War II soldiers with missile wounds to the brain, only few qualitative data are reported on the recovery of visual functions, and no attempts were made to treat the patients. In a later article, Teuber (1975) reported follow-up observations in 520 cases with known brain injuries incurred in World War II or in Korea or Vietnam. He confirmed Poppelreuter’s observation that vision can reappear in affected visual field regions.
In contrast to the diminishing interest in the study of recovery of vision after brain injury in man, there was growing interest among brain researchers on the effect of experimental lesions to brain structures subserving vision in animals. It was especially Klüver (1942) who studied the effect of experimentally induced localised lesions on vision in monkeys and found that bilateral occipital injury results in a profound but not total loss of processing of visual information. A “sufficiently long period of training” was, however, required before the monkey was able, for example, to locate objects in space. A famous single case study on a rhesus monkey, Helen, from whom the striate cortex was almost totally removed bilaterally, and who was studied intensively over a period of 8 years (Humphrey, 1974) revealed that the monkey could regain an effective, though limited, degree of visually guided behaviour by practice in her natural environments. Cowey (1967) and Weiskrantz and Cowey (1970) showed convincingly that practice can reduce the size of a cortical, but not a retinal, scotoma in monkeys, as defined by the animal’s ability to detect light targets. An even more complete and specific recovery of vision could be demonstrated by Mohler and Wurtz (1977). Deficits in detecting flashes in the cortical scotoma region and in the accuracy of saccadic eye movements directed towards them, disappeared 6 weeks after the lesions were performed. Again, systematic practice was necessary for recovery, which was mainly observed in the portion of the scotoma that had been subjected to practice. Thus, occipital lesions need not always result in an irreversible loss of vision, but systematic treatment is required for its return. Of course, the results obtained in animal studies cannot directly be transferred to patients. It has, for example, been shown that patients can also accurately respond to light stimuli presented in their cortical scotoma. For the demonstration of this phenomenon which has been coined “blindsight”, because patients are never aware of the presence of the target, special testing conditions are required, and typically patients show this capacity only after systematic practice (for a review, see Weiskrantz, 1986). It is still an open issue whether this “residual visual capacity” is due to recovery, represents residual functioning of spared visual cortex, or represents a visual function that is subserved by extrageniculostriate mechanisms that were not affected by injury. However, “blindsight” does not seem to reduce patients’ visual disability because they cannot make use of this sub- or unconscious visual function (Zihl, 1980). Therefore, although there is no doubt that in these cases, like in monkeys with cortical scotoma, visual processing takes place in the “blind” field region, nobody would go so far as to consider a patient showing blindsight “visually rehabilitated”. Likewise, the methods of practice used to uncover this type of visual processing cannot be considered appropriate for the treatment of patients with visual field loss due to occipital brain injury. Nevertheless, brain lesion research in primates has contributed substantially to the understanding of the recovery of visual function in humans. On the one hand, monkeys show considerable return of both elementary and complex visual function, although they typically need intensive and systematic training to overcome the deficit. On the other hand, these experiments show that total and irreversible loss of a particular visual ability is only to be expected if more than one structure of the neural network subserving this ability is injured (for a review, see for example, Frommer, 1978; Rothi & Horner, 1983; Stein, 1994). For example, Mohler and Wurtz (1977) have demonstrated that monkeys no longer recover from blindness after striate cortex injury when their ipsilateral colliculus superior is also destroyed. Similarly, patients with injury to the striate cortex and to the posterior thalamus may show a poorer treatment outcome concerning the compensation of their visual field defect than do patients with injury to the striate cortex only (Zihl, 1995a,b; see p. 59 for further discussion). Thus, the accurate control of the size of the lesion is a crucial issue in studying recovery of vision after brain injury.
The first and foremost question in rehabilitation after brain injury is whether there is any potential at all for recovery. If a particular visual function depends entirely on one single cortical structure, and if this structure is completely and irreversibly injured, then recovery of the affected visual function cannot be expected. Unfortunately, the definition of reversibility and irreversibility of brain injury is still an open issue, despite enormous improvements in brain imaging techniques. In cases of spontaneous recovery it is, of course, reasonable to assume that brain injury merely had reversible consequences (see Bosley et al., 1987). But does the opposite also always hold true, namely that the brain structure in question has really undergone irreversible injury when no spontaneous recovery occurs? A further similarly difficult, but important, question concerns the cortical representation of visual functions. “Functional specialisation” does not imply strict localisation of function. If it did, then injury to a particular cortical area would destroy the function in question completely and irreversibly. However, the situation is yet more complicated, as the two following case studies will demonstrate.
LM had lost most of her capacity to see motion following bilateral posterior brain injury due to sinus venous thrombosis (Zihl, von Cramon, & Mai, 1983; Zihl, von Cramon, Mai, & Schmid, 1991). She reported, however, to somehow “see” objects in motion, provided that (1) only one stimulus was moving; (2) the speed of the moving stimulus did not exceed 6deg/sec; and (3) objects were moving either horizontally or vertically. This “residual” perception of movement could either constitute incomplete injury to V5 (the “motion” area) or could be accounted for by other visual areas. The measurement of brain activity during the processing of moving visual stimuli in LM showed no evidence of activation of V5 in either hemisphere. Somewhat surprisingly, activation was observed in another visual area (V3) and in the superior parietal cortex (Brodmann’s area 7). Both areas, however, are not known to be “functionally specialised” to process visual motion signals and are not activated in normal subjects in the same experimental conditions, but are the likely candidates for the patient’s residual movement vision (Shipp et al., 1994). Thus, movement vision is possible without V5, although under extremely restricted conditions. This “residual” movement capacity did not improve over the years and could not be used by LM to substitute the role of her lesioned V5 which would have reduced her severe daily visual handicaps. Nevertheless LM has learned over the years to cope quite successfully with her severe visual disorder. She is able to manage by herself shopping, using public transportation means, keeping her flat and participating in social events as for example birthday parties. Her coping strategies are, however, mainly based on avoiding seeing objects or people in motion.
DF, a patient reported by Milner et al. (1991), suffered a severe visual deficit of form recognition following bilateral posterior brain injury due to asphyxia while taking a shower as a result of a faulty gas water heater. She had great difficulties in discriminating, for example, simple shapes and line orientations. Despite poor performance in these tasks, the patient had little difficulty in everyday visually guided activities such as opening doors, shaking hands, eating meals, reaching out accurately for and grasping objects differing in form, size, and orientation. The authors hypothesised that the preserved visuomotor ability may depend on routes still functioning from the occipital lobe, where the analysis of visual forms is performed, to neural mechanisms in the parietal lobe that control visually guided movements of the hand and fingers. Explicit visual form perception and recognition is therefore not a crucial prerequisite for an appropriate visual guidance for moving hand and fingers. In contrast to LM, who did not benefit from her residual visual motion perception, DF certainly could make use of her “residual” visual form-processing capacity regarding daily life activities.
Certainly, nobody would interpret the use of spared or substituted visual functions in these two cases as recovery, but these and similar observations underline the need for an accurate and detailed analysis of lost, impaired, spared, and substituted visual functions. Otherwise sparing or substitution of function could easily be confounded with recovery of function, especially if practice is required to reveal a spared or substituted visual capacity. Practice might be especially important in cases with denial of preserved vision (so-called negative Anton syndrome), and in cases with a reduction of initiative and self-generated activities due to concomitant depression or reduced motivation (e.g. Feibel & Springer, 1982; Richards & Ruff, 1989). The site and extent of a brain lesion differ, of course, among patients, but this may not be reflected by the (initial) severity of a single visual deficit or pattern of deficits. Recovery of visual function as well as functional improvement through compensation may, however, depend on the integrity of brain structures beyond the visual cortex and on fibre pathways interconnecting the various visual structures. Another difficulty arises because patients cannot (and must not) be kept in a “controlled” constant environment as animals can. Consequently, any kind of improvement of function can also be attributed to confounding “environmental” variables. Finally, how much time should a brain function be given to recover “spontaneously”, and how long should treatment be continued before one can reliably state that no (further) improvement can be expected? If systematic intervention is started at an early point in time and the function in question returns, partially or completely, a legitimate explanation would be that the improvement might just as well have taken place without systematic intervention. This is a serious argument, but not easy to dismiss—even if treatment is started several weeks or even months after the onset of a functional deficit and the deficit could therefore be assumed to be stable. One possibility of avoiding this problem is to use a control group to monitor the effect in the experimental group. This seems to be an ideal methodological approach, but again one is forced to deal with the problem of homogeneity of brain injury and the control of environmental influences. Of course, for the patient it is irrelevant why improvement has taken place as long as the outcome is beneficial; for the researcher it is not. There is agreement that the adoption of a method of treatment should be based on an underlying theoretical rationale and on the control for non-specific factors (e.g. motivation, emotional state, social support; Robertson, 1994). These factors may affect or enhance the improvement, although they have little or nothing to do with specific treatment procedures. It seems, therefore, that rehabilitation research in neuropsychology is not only a very laborious and difficult task, for which no really satisfactory methodology exists. At the same time it is also extremely risky because success cannot be guaranteed, even after a high expenditure of time, resources, and energy. However, reports on negative findings are just as important as reports on positive outcomes (Barlow & Hersen, 1985), not only on methodological grounds, but also for extracting criteria on which to base a valid decision about the efficacy of a particular treatment procedure. Of course, when developing and proving new methods of practice, one cannot always predict the potential significance to rehabilitation. What is possible, however, is to design a priori the development and evaluation of treatment methods, to decide on patient eligibility criteria, and to select measures of outcome in the context of behavioural benefit (Baddeley, Meade, & Newcombe, 1980). This is not only important from the viewpoint of rehabilitation, which implies functional improvements for the sake of more independence and higher quality of life, but also for the motivation of patients. The earlier the patient is aware of an improvement in everyday life activities, the higher will be their motivation to co-operate and the earlier the patient will become an expert for the specific individual difficulties, and how to cope with new, unknown and unfamiliar conditions.
Although the number of publications on neuropsychological rehabilitation is increasing and a special journal has been founded for this discipline in 1991 (Neuropsychological Rehabilitation), this field still has not been the focus of neuroscientific research. This is a pity because the study of the recovery of brain function and of mechanisms of substitution and compensation is not only of importance for neuropsychological rehabilitation, but also can contribute substantially to the understanding of the functional organisation and reorganisation of the brain and of brain plasticity. The better we understand the relationships between brain and behaviour, especially in “pathological” conditions, the more success we shall have in the development of rehabilitation methods, and the more the patient will benefit. Concerning visual deficits following brain injury, there is meanwhile agreement that rehabilitation is important because vision probably represents the most important sensory system in humans and is required for the guidance of many motor activities. Thus the understanding and accurate identification of visual deficits and the implementation of specific treatment strategies is important to maximise functional independence of patients (Anderson & Rizzo, 1995; Raymond, Bennett, Malia, & Bewick, 1996).
This book deals with the rehabilitation of visual deficits after acquired brain injury. In the following chapters the main cerebral visual disorders will be described, observations of spontaneous recoveries reported, and methods of treatment outlined. To facilitate understanding, each section will begin with a brief description of the particular visual deficit(s) or disorder(s), and consequences for the patient in terms of disability and handicap. Observations of spontaneous recoveries and the rationale and outcome of studies on the effect of treatment will follow. Many observations stem from investigations of the author; some of them have been published, others have not. For some fields of treatment, results of larger groups of patients were available; for others, observations of single cases are presented. Some of the studies, for example on visual field disorders, were systematically prepared and the methodology applied fulfils, at least in part, the criteria required for such studies. Where a sufficiently large number of patients was available, a within-subject repeated-measures design was used, which permits the direct comparison of pre- and post-treatment performance in a given test for each patient. This design is easier to carry out in the context of rehabilitation research than randomised groups studies, and in a...
Table of contents
Citation styles for Rehabilitation of Visual Disorders After Brain Injury
APA 6 Citation
Zihl, J. (2021). Rehabilitation of Visual Disorders After Brain Injury (1st ed.). Taylor and Francis. Retrieved from https://www.perlego.com/book/2555639/rehabilitation-of-visual-disorders-after-brain-injury-pdf (Original work published 2021)
Chicago Citation
Zihl, Josef. (2021) 2021. Rehabilitation of Visual Disorders After Brain Injury. 1st ed. Taylor and Francis. https://www.perlego.com/book/2555639/rehabilitation-of-visual-disorders-after-brain-injury-pdf.
Harvard Citation
Zihl, J. (2021) Rehabilitation of Visual Disorders After Brain Injury. 1st edn. Taylor and Francis. Available at: https://www.perlego.com/book/2555639/rehabilitation-of-visual-disorders-after-brain-injury-pdf (Accessed: 15 October 2022).
MLA 7 Citation
Zihl, Josef. Rehabilitation of Visual Disorders After Brain Injury. 1st ed. Taylor and Francis, 2021. Web. 15 Oct. 2022.