
- 264 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Adoptive Cellular Immunotherapy of Cancer
About this book
This volume presents the most complicated and powerful cancer biotherapies developed. It provides an overview of human immune system function and the mechanisms by which adoptive cellular immunotherapies (ACI) harnesses the activity. The volume provides a vision on the developments in ACI.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Adoptive Cellular Immunotherapy of Cancer by H. C. Stevenson in PDF and/or ePUB format, as well as other popular books in Medicina & Inmunología. We have over one million books available in our catalogue for you to explore.
Information
1
Adoptive Cellular Immunotherapy of Cancer: An Overview
National Cancer Institute, Bethesda, Maryland
A variety of distinct strategies to boost the immune system function of the cancer patient in an attempt to treat malignancy have been developed over the past several decades (1–6). It is clear from reviewing the immunological research of the past 100 years that the human immune system is capable of fulfilling its visualized objectives of eliminating nonself invaders from the body; this includes such distinct life forms as viruses, bacteria, and fungi. As will be detailed in this text, compelling evidence has recently been accumulated to indicate that host cells that have undergone malignant transformation not only behave as nonself invaders (attempting to take over the rest of the host), but they also bear chemical markers of malignant transformation (tumor antigens) that allow these cells to be “recognized” by the immune system; such malignantly transformed cells can also be destroyed by host immune system cells. The decade of the 1980s has focused extensively on the development of a new generation of cancer bio-therapies—new immunotherapeutic techniques designed to “up-regulate” human immune system function in an attempt to irradicate malignant cells from cancer patients.
Current biotherapy approaches to cancer treatment are diagramed in Figure 1. From a functional perspective, three different types of bio therapy research strategies are now being explored. One strategy involves the ex vivo manipulation of the cancer patient’s plasma in an effort to remove blocking factors or to add tumoricidal factors to the plasma before the plasma reinfusion. Such therapies usually require a centrifugation separation step to remove plasma from the other blood elements, followed by passage of the plasma over a separate column device such as is employed in staphyloccocal protein A column therapy (7). A more frequently employed biotherapy approach involves the direct in vivo treatment of cancer patients with biological response modifiers (BRM) in an attempt to utilize these agents to directly stimulate the failing immune system within the cancer patient’s body. Such BRM include the interferons (IFN), the interleukins (IL), colony-stimulating factors (CSF), monoclonal antibodies (MAb) and other immunopotentiating agents, such as muramyldipeptide (MDP) and Corynebacterium parvum (C. parvum) (4, 8, 9). (More details about certain of these BRMs will follow in this chapter.) A final functional classification of current biotherapy focuses on the ex vivo activation of cancer patient’s leukocytes with BRM in an attempt to expand their numbers or to increase their antitumor capabilities, or both; this approach has been termed adoptive cellular immunotherapy (ACI). Adoptive cellular immunotherapy is usually initiated with the performance of a cytapheresis procedure to separate the white blood cells (WBC) from the plasma and the red blood cells (RBC); the plasma and RBC are returned to the patient immediately; alternatively, the WBC may be isolated from patient tumor specimens directly. The WBC undergo further processing, including their incubation with BRM designed to augment or to increase their tumor-killing capabilities. These activated WBC are then reinfused back into the patient. The purpose of this volume is to present our current understandings of the mechanics and mechanisms of action of the ACI protocols currently being performed.
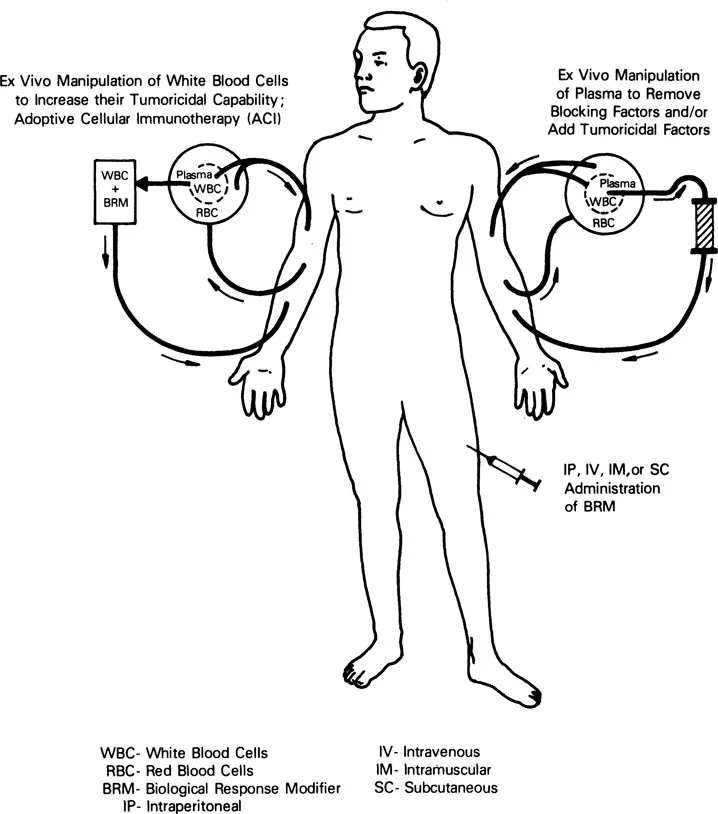
Figure 1 Representation of the functional mechanics involved in the performance of the three major types of cancer bio therapy: (a) Adoptive cellular immunotherapy (ACI), (b) ex vivo manipulation of patient plasma to remove blocking factors or add tumoricidal factors, and (c) in vivo administration of BRM.
THE ANTITUMOR ACTIVITIES OF THE HUMAN IMMUNE SYSTEM
Within the past two decades, we have gleaned many insights into the overall operation of the human immune system and the potential applicability of this system to the treatment of cancer. As shown in Figure 2, the immune system mechanisms that are felt to be operative in the elimination of tumor cells from the body are rather straightforward. There are four basic cell types whose function has been associated with antitumor cell immunity. The B-lymphocytes secrete protein factors [immunoglobulins (Ig)] into the blood plasma, which have the capability of identifying and binding to targets that bear specific molecular markers that they can recognize. From a functional perspective, each individual possesses enough different types (clones) of B lymphocytes (and thus enough different Ig types) to identify most nonself invaders; this specific component of humoral (plasma-borne) immunity is a potent mechanism for identifying and labeling nonself invaders. It is noteworthy, however, that Ig-labeled target cells, in and of themselves, perform in a relatively unimpeded fashion; that is, additional factors are required for Ig-coated targets (such as tumor cells) to be destroyed. The two dominant mechanisms whereby Ig-coated targets are cleared from the body are through the complement cascade (the nonspecific component of humoral immunity) and the antibody-dependent cell-mediated cytotoxicity (ADCC) mechanisms (cellular mechanisms reviewed below). The complement proteins are a series of proteins that exist in an inactive state in the blood plasma under normal conditions. However, upon encountering an immunoglobulin (IgG or IgM)-coated target, these plasma proteins assemble into a trocarlike mechanism that is capable of lysing nonself invader cells (including tumor cells). The complement proteins are chiefly secreted by the mononuclear phagocyte series (blood monocytes). Since the advent of MAb technology (4), great attention has been focused on the protein products of B lymphocytes and the application of these Ig proteins to the treatment of cancer. For this reason, neither the Ig proteins nor their cellular factories (the B lymphocytes) will be discussed further in this volume, except to mention that the B lymphocytes are subject to activation and regulation by a variety of protein signal molecules—the BRM-chemotherapy agents, and other chemicals that can influence their function. These modulating agents will be reviewed relative to the other leukocyte subsets that participate in the overall immune response to cancer.
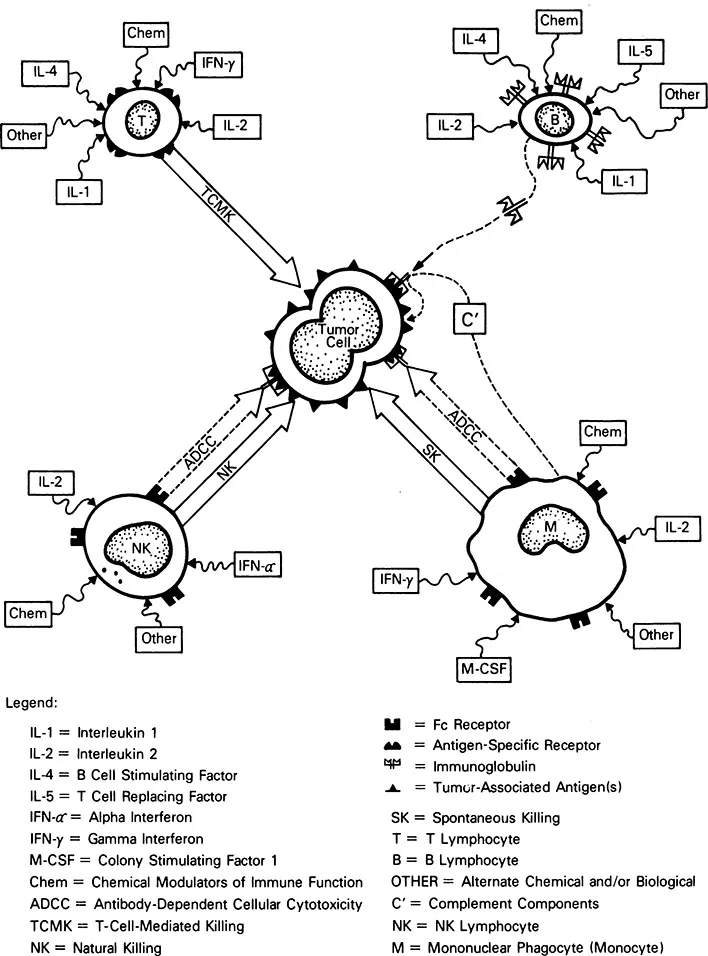
Figure 2 Representation of the potential mechanisms of action whereby B, T, and NK lymphocytes and mononuclear phagocytes (monocytes and macrophages) may eliminate tumor cells.
The two remaining lymphocyte subsets have been harnessed for ACI. They are the natural killer (NK) lymphocytes and the T lymphocytes. The T lymphocytes exist in clones as do the B lymphocytes; in contrast with B lymphocytes, they do not function by synthesizing proteins for transport through the blood stream to mediate tumor cell killing but, rather, they physically travel to the site of tumor cell invasion and locally arrange for the destruction of the tumor cells. As shown in Figure 2, T lymphocytes possess antigen-specific receptors, and each T-lymphocyte clone has the capacity to recognize (and potentially destroy) a tumor cell carrying complementary marker molecules on its membrane (tumor-associated antigens). Given the total spectrum of T-lymphocyte clones present in the body, it appears that each individual possesses the total range of T-lymphocyte clones required to eliminate most types of tumor cells (1). The T lymphocytes destroy tumor cells through their antigen-specific receptors. Such killing is termed T-cell-mediated killing (TCMK) and is antigen-specific; that is, each T-lymphocyte clone can eliminate only a very narrowly related group of tumor cells bearing the same tumor-associated antigen. Moreover, TCMK by T lymphocytes is restricted by the major histocompatibility (MHC) molecules; that is, tumor cell killing by T-lymphocyte clones requires identity between the MHC molecules found on the surface of the tumor cell and specific MHC receptors found on the surface of the T lymphocyte. T-cell-mediated killing can be modulated by a variety of influences. These include many BRMs, such as interleukin-1 (IL-1), interleukin-2 (IL-2) and interleukin-4 (IL-4), and inter-feron-γ (IFN-γ) (reviewed later), as well as chemotherapy agents and other chemicals. Some researchers believe that tumor-infiltrating lymphocyte (TIL) therapy is mediated by activated antigen-specific T-lymphocyte clones; however, this particular ACI topic is the subject of a substantial amount of controversy that is excellently summarized in Chapter 9. The developers of the tumor-derived activated cell (TDAC) therapy believe that they infuse activated antigen-specific T lymphocytes into their patients as reviewed in Chapter 10.
The natural killer (NK) lymphocyte is the third well-characterized lymphocyte subset that has antitumor cell reactivity. In contrast with the T lymphocyte, the NK lymphocyte bears no evidence of clonality; that is, a single type of cell appears to be capable of destroying a wide range of human tumors. This large-volume lymphocyte subset has distinctive granules in its cytoplasm and also has been termed the large granular lymphocyte (LGL; 10, 11). Natural killer lymphocytes have two distinct mechanisms for the destruction of tumor cells. One is a mechanism known as antibody-dependent cellular cytotoxicity (ADCC). This cytotoxicity occurs when special membrane receptors (Fc receptors) on the surface on the NK lymphocyte bind to the terminal (Fc) portion of Ig molecules (IgG) that have bound to the tumor-associated antigens found on the surface of tumor cells. The Ig molecule then functions as a ligand between the NK lymphocyte and the tumor cell and promotes the rapid destruction of the tumor cell. Alternatively, NK lymphocytes can kill tumor cells independent of Ig; this killing is termed natural killing (NK). The precise molecular marker that promotes natural killing is not now known; however, it appears to be a glycolipid molecule (ganglioside) that appears on virtually all malignantly transformed cells. The baseline natural-killing capability of NK cells can be upregulated by a variety of BRM including interferon-α (IFN-a) and IL-2 (reviewed later); it can also be modulated by chemotherapy agents and other chemicals. The lymphokine-activated killer lymphocyte (LAK) can be generated by upregulating the tumoricidal activity of NK lymphocytes by the in vitro incubation of this cell type with the BRM, IL-2; however other LAK precursor cells may exist. This issue is addressed in Chapter 2.
The third subset of WBC that can be harnessed for ACI trials is not a lymphocyte, but rather is from the mononuclear phagocyte series, chiefly in the form of blood monocytes. Like the NK lymphocytes, monocytes possess two independent mechanisms for destroying tumor targets. Mon...
Table of contents
- Cover
- Half Title
- Series Page
- Title Page
- Copyright Page
- Preface
- Contributors
- Contents
- 1 Adoptive Cellular Immunotherapy of Cancer: An Overview
- 2 Lymphokine-Activated Killer Lymphocytes: Evidence for Regulation of Induction and Function by Multiple Cytokines
- 3 Lymphokine-Activated Killer Lymphocytes: National Cancer Institute Clinical Trials
- 4 Lymphokine-Activated Killer Lymphocytes: Extramural Clinical Trials
- 5 Lymphokine-Activated Killer Lymphocytes: Biotherapeutics Clinical Trials
- 6 Lymphokine-Activated Killer Lymphocytes: LAK and Interleukin-2 in the Treatment of Malignancies of the Central Nervous System
- 7 Activated Killer Monocytes: Preclinical Model Systems
- 8 Activated Killer Monocytes: Use in Clinical Trials
- 9 Tumor-Infiltrating Lymphocytes: Antigen-Specific Killer T-Lymphocytes or Activated Natural Killer Lymphocytes?
- 10 Tumor-Derived Activated Cells: Culture Conditions and Characterization
- 11 Clinical/Technical Challenges in Adoptive Cellular Immunotherapy: The Role of Cytapheresis
- 12 Clinical/Technical Challenges in Adoptive Cellular Immunotherapy: In Vitro Culture Techniques
- 13 Clinical/Technical Challenges in Adoptive Cellular Immunotherapy: Role of Nurses, Technicians, and Other Support Personnel
- 14 Adoptive Cellular Immunotherapy of Cancer: Future Perspectives
- Index