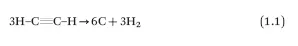Graphite is one of the most abundant allotropes of carbon in nature and the most used in our day to day through its best-known application as part of pencils, however, we rarely mention its electrical properties, which allow it to be used as an electrode. The properties of graphite are due to the fact that it is made up of carbon atoms with hybridization sp2 constructed in the form of 2D sheets, which are stacked on top of each other and joined by van der Waals forces. Additionally, this carbon allotrope is one of the cheapest and easy to modify, which makes it an ideal component for manufacturing of low-cost carbon composites.
1.2.1 Composites of Graphite (Graphite Oxide) with Carbon Nanotubes
Carbon nanotubes (CNTs) can be divided into two types: single-walled carbon nanotubes (SWCNTs) and multi-wall carbon nanotubes (MWCNTs). Regardless of the form in which the CNTs are, they are the nanostructures that have attracted the most attention of researchers along with graphene. For this reason, graphite/CNT composites are one of the most commonly found in literary reports. Some of the most promising applications where these composites have been implemented are in electroanalysis of bioactive molecules, battery electrodes, supercapacitors, sensors etc.12 The synergies that exist between the 2D structures of the graphite sheets and the 1D structures of CNT allow the resulting composite to have improvements in some of its properties such as electrical conductivity, adsorption capacity, mechanical and tribological properties.3 Furthermore, graphite/CNT composites can be manufactured using a wide variety of methods, among which chemical vapor deposition (CVD) stands out, as it is the most common method found in the literature.
The selective synthesis of SWCNTs/graphite composites on nickel foam was carried out in different ways, the first by CVD from acetylene gas a carbon precursor (eqn (1.1)).4 Other synthesis methods are also available, for instance, graphite fiber composites with SWCNTs (this process could be scaled up after additional improving efforts and adaptation to the existing manufacturing process) were obtained by SWCNTs air-spraying onto the surface of graphite/epoxy prepreg.5
SWCNTs/graphite composites out-of-plane electrical conductivity was improved by 144% for 2 wt.% SWCNT samples compared to samples without SWCNTs. The composite of treading CNTs and coated graphite was obtained as a result of the pyrolysis of CNT/polyaniline composites at 1500 °C, presenting a specific orientation relationship between the CNTs axis and graphene layers,6 with an angle of 110° between them. This synthesis method can be applied to fabricate high-performance carbon materials via the alignment of graphene layers.
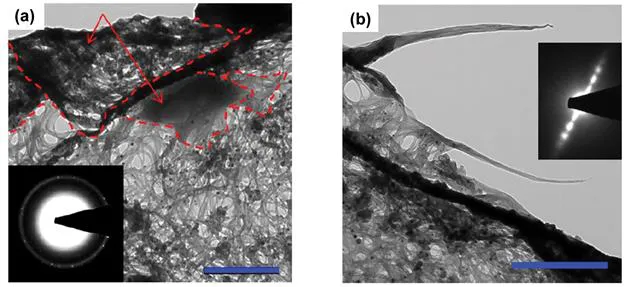
Some reports established several intriguing differences between graphite oxide (GrO) and reduced graphite oxide (rGrO) behavior in carbon nanotubes, which have direct effects on the electrical conductivity of the composite.7 In particular, the presence of electrically insulating GrO within a SWCNT network (Figure 1.1) strongly enhances electrical conductivity, meanwhile rGrO, even though electrically conductive, suppresses electrical conductivity, revealing the “indirect” role of the oxide groups. These groups, being in GrO within the SWCNT/GrO composite act through electronic doping of metallic SWCNTs. The following two factors controlling electronic transport were proposed:
- a) In SWCNT networks: high intrinsic conductivity of SWCNT versus poor coupling between nanotubes.
- b) In rGrO networks: good coupling between the sheets versus poor intrinsic conductivity within the sheet.
Figure 1.1 Transmission electron microscopy (TEM) micrographs of the CNT/GrO composite seen from the top (a) and from the side (b), in addition, the backings show the diffraction of electrons from both perspectives.7 Reproduced from ref. 7 with permission from Elsevier, Copyright 2014.
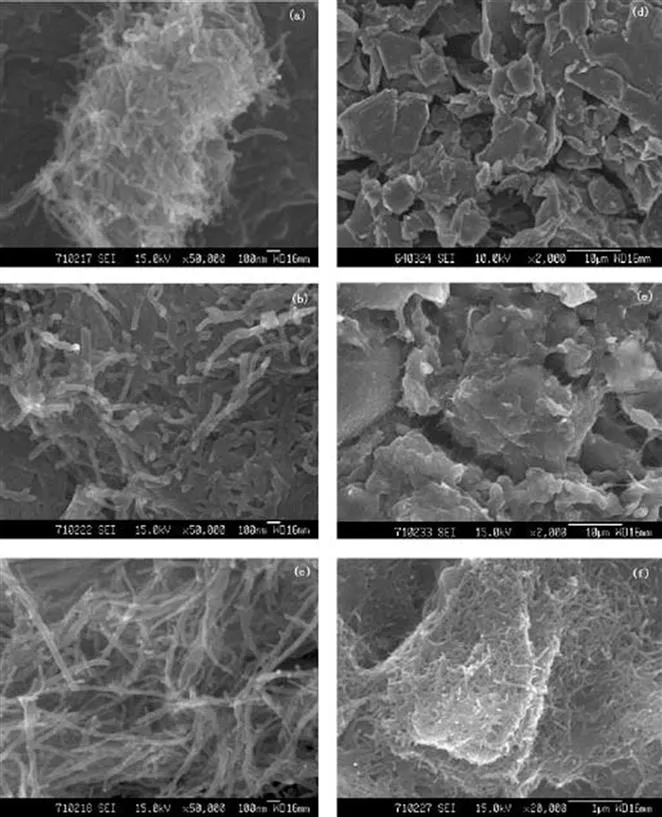
Graphite/CNT composites alone, as well as their derivatives with metal oxides, are mainly used for the manufacture of Li-based storage equipment and supercapacitors. In one of them, the graphite/CNT compound (Figure 1.2), the CNTs are dispersed evenly on the surface of the graphite sheet when they are mixed together.8 For a three-phase-containing hybrid (Figure 1.3), consisting of carbon nanotubes (CNTs), and commercial graphite particles, and graphene oxide sheets, prepared using ultrasound with strongly oxidizing reagents such as KMnO4 and H2O2, it was revealed9 that, after compositing with graphite and CNTs, the graphene oxide maintains its typical wrinkled paper-like structure. The graphite particle and CNTs are covered by GO sheets, and the CNTs are randomly aligned to form a conductive bridge. This composite was found to have a very high reversible Li-storage capacity of 1172.5 mA h g−1 at 0.5C (1C = 372 mA g−1), exceeding the theoretical sum of capacities of the three ingredients. This composite can be directly used as a binder-free anode material, possessing excellent electrochemical characteristics.
Figure 1.2 Scanning electron microscope (SEM) micrographs of graphite, CNT and their composite. (a) CNT, (b) CNT treated at 200 °C in air, (c) CNT treated at 200 °C in vacuum, (d) graphite, and (e and f) CNT/graphite composite with 5 wt.% CNT treated at 200 °C in vacuum.8 Reproduced from ref. 8 with permission from Elsevier, Copyright 2008.
Figure 1.3 SEM micrographs of (a) graphite, (b) CNT, (c) graphene oxide, and (e) graphene oxide/graphite/CNTs with the conductive additive composite (GGCC). TEM images of (d) graphene oxide and (f) GGCC composite.9 Reproduced from ref. 9 with permission from Elsevier, Copyright 2014.
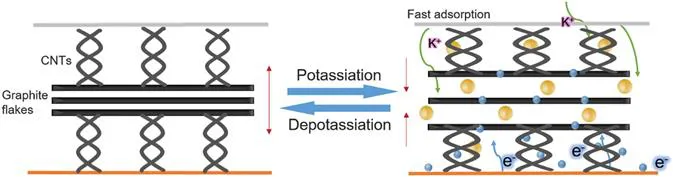
However, despite the good conductivity that graphite/CNT composites have presented, these materials present some long-term drawbacks in the manufacture of batteries, the two most relevant problems are structural pulverization and the instability of the solid electrolyte interface,1 which are the result of constant volume changes during charge/discharge cycles. One way to avoid structural spraying problems is by adding spacers between the graphite sheets (Figure 1.4). In this case, a layer of CNTs of micrometric lengths are grown, which are interlaced on the surfaces of the graphite flakes, this allows the CNTs to function as an effective conductive medium and as a volumetric change buffer, due to these structural characteristics, the composite after being subjected to 1500 charge/discharge cycles, exhibits a reversible capacity of 234 mA h g−1 at 2 A g−1 and it retains 97% of its capacity, which is better than graphite flakes alone.1
Figure 1.4 Scheme that exemplifies how CNTs work between the graphite sheets of the composite in the charge/discharge processes.1 Reproduced from ref. 1 with permission from Elsevier, Copyright 2019.
As an example of a complex composite of “graphite/CNTs/metal oxide”, we have an ultra-thin 3D ...