
- 800 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Analysis of Addictive and Misused Drugs
About this book
Examines the chromatographic and nonchromatographic methods available to identify, measure, and screen for nonmedical drug use, highlighting the latest technologies in immunochemical analysis, biosensors, thinlayer gas chromatography, high-performance liquid chromatography, and capillary electrophoresis. A comprehensive alphabetic listing of over 400 controlled-use drugs is provided.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Analysis of Addictive and Misused Drugs by John A. Adamovics in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Analytic Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Topic
Physical SciencesSubtopic
Analytic Chemistry1
Enzyme Immunoassays
Hycor Biomedical Inc., Irvine, California
I. INTRODUCTION
Immunoassay is an analytical technique that relies on the specificity between an antibody and its complementary antigen to measure the concentration of either of these two species in a reaction mixture. The term “immunoassay” usually refers to a quantitative method based on the reversible binding of an antibody (Ab) to an antigen (Ag) according to the law of mass action:
The strength of the bond between the antigen and the antibody is the affinity of the antibody, a property of the antibody that determines the extent to which the AbAg complex dissociates. Immunoassays are widely used for the detection of a broad range of analytes of clinical interest. Their use is widespread because most analytes are either antigens or antibodies and antibodies having both high-specificity and high-affinity constants can be developed against them. Immunoassay methods can be divided into several groups according to the assay conditions, test sample, assay system, type of analysis, and so on [1]. The assay system can be divided into two types, based on the method used to detect the reaction between antigen and antibody. These two types are distinguished by the use of a labeled or non labeled reactant.
The use of different labels in the development of immunoassays has resulted in a broadly accepted nomenclature based on the choice of label used. Radioimmunoassay, which uses radioisotopes as a marker, was developed by Yalow and Berson in 1959 [2]. The variety of nonisotopic immunoassays currently available began with the development of an enzyme immunoassay by Van Weeman and Shuurs in 1971 [3]. Numerous labeled methods are available (see Table 1). This chapter is dedicated exclusively to those methods that make use of enzymes as markers.
| Radioimmunoassay |
| Enzyme immunoassay |
| Fluorescence immunoassay |
| Luminescence immunoassay |
| Spin immunoassay |
| Particle immunoassay |
II. ENZYME IMMUNOASSAYS
Enzyme immunoassays can be classified into two different types of assays. These are commonly referred to as homogeneous and heterogeneous enzyme immunoassays (ElA). Heterogeneous immunoassays are those that require separation of antigen-antibody complexes from free antigen and antibody to determine the activity in one or both of the separated fractions. Such heterogeneous assays are based on the same principles as those used in radioimmunoassays and require a solid phase as is found in the popular enzyme-linked immunosorbent assay (ELISA) [4,5]. In contrast to heterogeneous enzyme immunoassay, homogeneous enzyme immunoassay requires no separation. One of the earliest homogeneous enzyme immunoas says was based on EMIT technology (enzyme-multiplied immunoassay technique) [6,7]. A number of analogs to EMIT have been developed. These include fluorescence polarization immunoassay [8] and cloned enzyme donor immunoassay (CEDIA) [9]. The homogeneous immunoassay technology makes it possible to automate these assay formats and apply them to routine clinical analyzers, as in EMIT and CEDIA. Because neither washing nor separation is involved, however, homogeneous immunoassays suffer from limitations in sensitivity. To reduce interferences caused by the specimen, the sample is frequently diluted, thus imposing on the assay system the need for antibodies of the highest affinity. Yet another limitation of homogeneous immunoassays is that high-molecular-weight analytes may interfere with signal generation when coupled to the label. In general it can be said that ELISA may be used for the detection of any antigen, whereas the application of EMIT is probably limited to the assay of low-molecular-weight haptens, such as drugs.
Enzyme immunoassays may be classified as either competitive or non-competitive?, depending on whether the technique involves a reaction step in which unlabeled and labeled antigen compete for a limited number of antibody sites (competitive assay) or whether the antigen (or antibody) to be measured is first allowed to react with antibody (antigen) on a solid phase followed by measurement of the binding of enzyme-labeled immune reactant (noncompetitive assay). More simplistically, the two formats can be distinguished by the use of limited (competitive) or excess (noncompetitive) reagent.
A variety of different enzymes have been used in immunoassays and are listed in Table 2. The most frequently used enzymes in immunoassays are horseradish peroxidase (HRP) and alkaline phosphatase. The properties of an enzyme that make it suited for use in immunoassays include those shown in Table 3.
| Alkaline phosphatase |
| Galactosidase |
| Horseradish peroxidase |
| Glucose-6-phosphate dehydrogenase |
| Lactamase |
| Urease |
| Readily conjugated |
| Readily available and inexpensive |
| High turnover rate |
| Good stability in solution |
| Stable substrate |
A. Competitive Assays
The principal features of the competitive assay for an ELISA using antigen-enzyme conjugate are illustrated in Fig. 1. The first step in this assay is the attachment of an appropriate amount of antibody to a solid phase. To the solid support is added a solution containing a known amount of enzyme-labeled antigen together with a standard antigen or unknown sample. The reaction mixture is then incubated to allow the antigen-antibody to attain equilibrium. After washing, substrate is added and the enzyme activity is determined following an incubation period. The concentration of the product of the reaction is inversely proportional to the concentration of the standard or test antigen added.
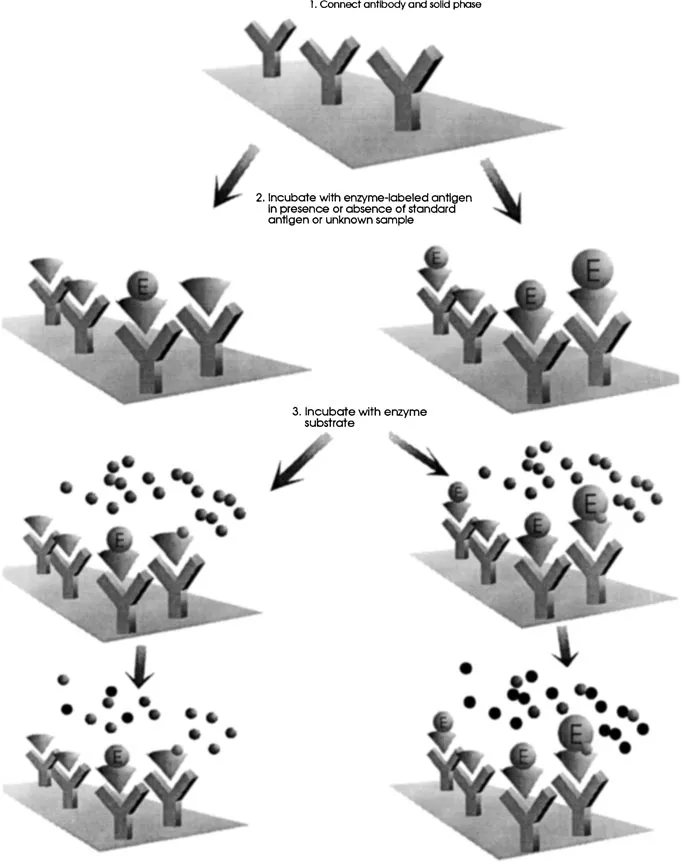
Figure 1 Competitive solid-phase immunoassay.
The assay format for EMIT using antigen-enzyme conjugate uses a fixed amount of hapten-enzyme conjugate. This is incubated with a fixed amount of hapten-specific antibody and a variable amount of free hapten [10]. The antibody modifies the enzyme activity upon reaction with one or more haptens on the enzyme. In this assay no separation is required and the enzyme reaction rate rather than an end point is measured. Since the concentration of immunoreactants in competitive assays is low, the specificity of such assays is generally good.
B. Noncompetitive Assays
The most familiar form of noncompetitive assay is the “sandwich” assay. The analyte is captured by a solid-phase-bound antibody, which is in excess (Fig. 2). After washing, the immobilized antibody-antigen complex is incubated with an excess of enzyme-labeled antibody. Upon removal of unreacted label by washing the solid phase, the label is detected by addition of an enzyme substrate. In this assay the concentration of the product of the enzyme reaction is directly proportional to the concentration of the standard or test antigen. Most noncompetitive immunoassays are of the sandwich type, implying that the analyte must be able to bind to two antibodies simultaneously. This excludes small molecules, such as drugs and steroids. It was recently reported, however, that noncompetitive immunometric techniques have been developed with high sensitivity for molecules of less than 1500 daltons [11–13].
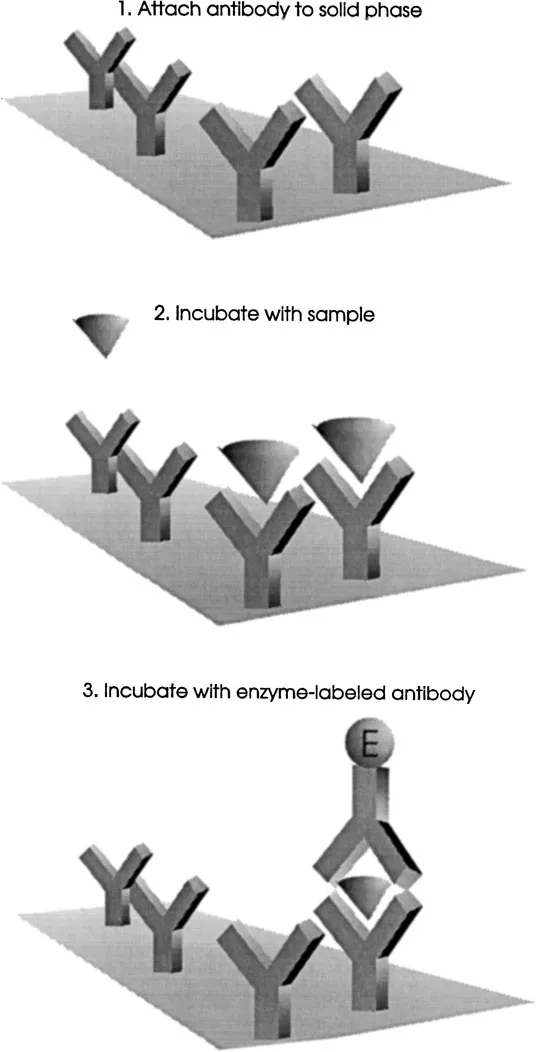
Figure 2 Noncompetitive solid-phase immunoassay.
III. DRUGS OF ABUSE TESTING
In 1988 the U.S. Department of Health and Human Services issued “Mandatory Guidelines for Federal Workplace Drug Testing Programs” [14]. These, the National Institute on Drug Abuse (NIDA) guidelines established federal standards for conducting urine drug testing on federal employees and certification standards for laboratories that would test these specimens. By the end of 1988, 10 laboratories had received certification from the NIDA, which administers the U.S. Department of Health and Human Services program and issues certificates. Currently the number of certified laboratories is close to 90.
A. Illegal or Abused Drugs
Presidential Executive Order 12564 defines illegal drugs as those included in Schedule I or II of the Controlled Substances Act, but not when used pursuant...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Preface
- Contents
- Contributors
- 1. Enzyme Immunoassays
- 2. Biosensors
- 3. Thin-Layer Chromatography Using the Toxi-Lab System
- 4. Reversed-Phase High-Performance Liquid Chromatography Analysis of Drugs of Forensic Interest
- 5. High-Performance Liquid Chromatography Using Unmodified Silica with Polar Solvents
- 6. Analysis of Seized Drugs by Capillary Electrophoresis
- 7. Thin-Layer Chromatographic Screening and Gas Chromatographic/Mass Spectrometric Confirmation in the Analysis of Abused Drugs
- 8. Robotics and the Analysis of Drugs of Abuse
- 9. Drug Testing of Athletes
- 10. Drug Analysis in South America
- Appendix: Supplementary Applications and Information
- Index