O. OUARI* AND D. GIGMES*
1.1 Brief History
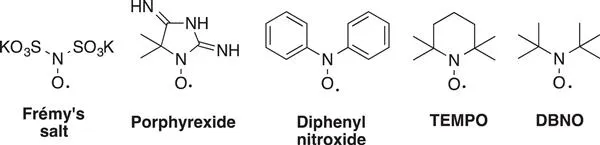
Nitroxides, also named aminoxyl or nitroxyl radicals, constitute a family of versatile organic radical species with rich and diverse properties and reactivity.1,2 Nitroxides belong to an important class of compounds finding a wide range of applications related to the fields of organic chemistry, physical-chemistry, material science, biology and medicine. Nitroxide compounds have a long history that started in 1845 with the first nitroxide prepared by E. Frémy.3 Known as Frémy's salt (Scheme 1.1), this radical was initially obtained by oxidation of potassium disulfonate hydroxylamine with lead dioxide and then by reaction of sodium nitrite with sodium bisulfite. The first organic nitroxide, so-called porphyrexide, was then prepared in 1901 by Piloty and Schwerin (Scheme 1.1).4 Later, in 1914, a nitroxide bearing two aryl groups on the nitrogen atom was obtained for the first time (Scheme 1.1) by Wieland et al.5 In 1959, the synthesis of the first cyclic N,N-di-alkyl nitroxide, namely 2,2,6,6-tetramethylpiperidinyl-1-oxyl, better known under the name of TEMPO, was described by Lebedev and Kazarnovskii (Scheme 1.1).6 Since this report, N,N-dialkyl nitroxides have taken on increasing importance over the years. In 1961, the synthesis of non-cyclic di-tert-butylnitroxide was described by Hoffmann and Henderson (Scheme 1.1).7
Scheme 1.1 Structures of the early nitroxides.
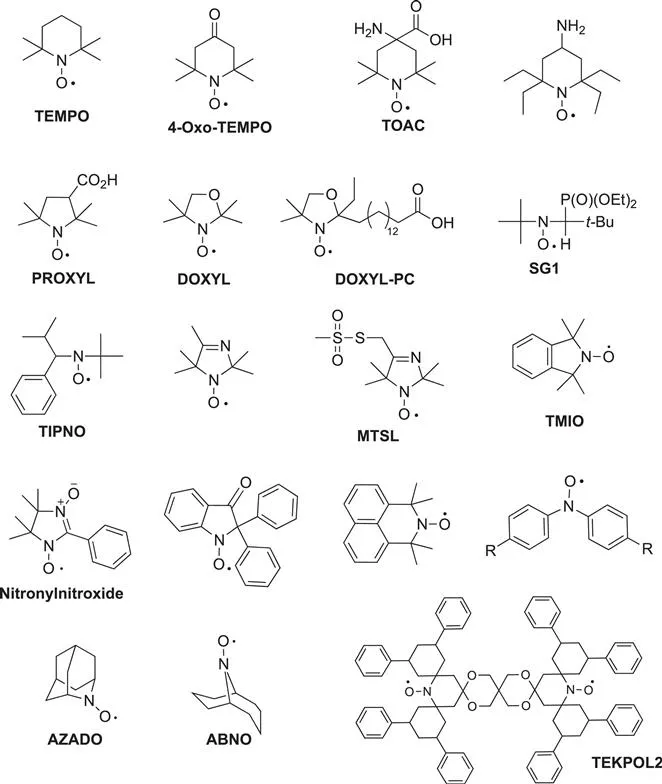
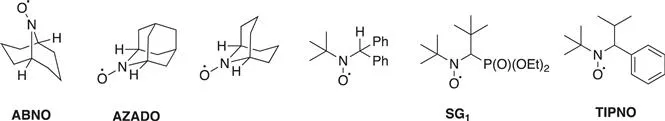
It has to be mentioned that since the 1960s the development of both efficient methodologies in organic chemistry and electron spin resonance (EPR) spectroscopy has been extremely beneficial for the development of the nitroxide field (Scheme 1.2).8 Several nitroxide structures such as TEMPO, TOAC, PROXYL, MTSL, DBN, TMIO, AZADO, ABNO and DOXYL are now commercially available and many more have been already described.2,9,10
Scheme 1.2 Some examples of the most common nitroxide structures.
The term stable radical11 implies that the nitroxide compounds can be safely handled and stored under ambient conditions, without any precaution of temperature, dioxygen (O2) or moisture exposition. The unusual radical stability and the possible reactions proceeding on the their structure with conservation of the radical properties were early recognized to be a key feature of nitroxide compounds12–14 explaining the widespread research attention on their reactivity and applications.
1.2 Nitroxide Reactivity
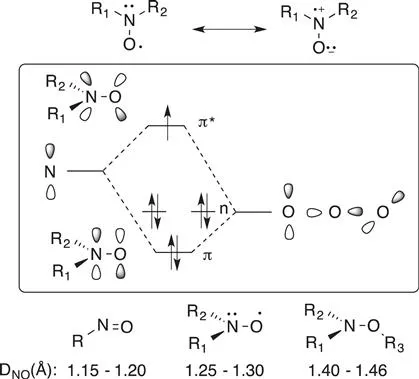
The exceptional properties of nitroxides result from the presence of a three-electron π bond in the aminoxyl group (N–O˙). This bond consists in the overlapping of the 2pz orbitals of the nitrogen and oxygen atoms. Hence, the electronic structure of the N–O˙ group is represented by the contribution of two resonance forms A and B (Scheme 1.3).
Scheme 1.3 Representation of two resonance structures of nitroxides, a schematic representation of the n, π, π* orbitals on the NO˙ group and the bond length of nitroso, nitroxide and alkoxyamine compounds.
Due to the presence of a single electron in the π* orbital (SOMO), the bond index of the N–O bond is 1.5. In addition, the value of the bond energy (≈418 kJ mol−1) and the bond length (1.25 Å<dNO<1.30 Å) are between those of a single NO bond of an hydroxylamine or alkoxyamine (N–OH≈222 kJ mol−1 and 1.43 Å, respectively) and those of a NO double bond of a nitroso compound (≈606 kJ mol−1 and 1.20 Å, respectively).
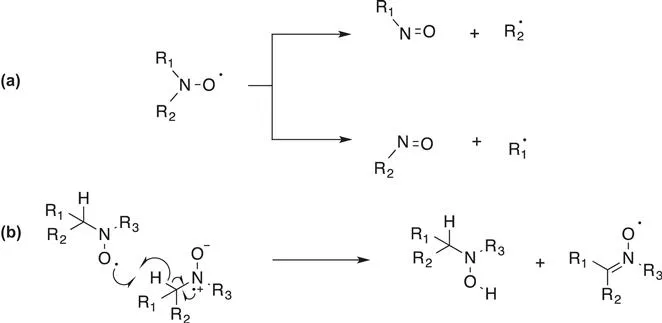
In dialkylnitroxides (R1=R2=alkyl) the spin density is essentially distributed between the nitrogen and oxygen atoms with a slightly larger value on the oxygen atom. The delocalization of the single electron of an aminoxyl group corresponds to an energy gain of approximately 100 kJ mol−1. However, the formation of an O–O bond leads to an energy gain of 170 kJ mol−1. Consequently, the dimerization of nitroxides is not thermodynamically favored because it does not correspond to a gain in bonding electrons. Typically, nitroxides having one or more hydrogen atoms on the α-carbons (Cα) of the nitrogen atom are not stable due to a disproportionation reaction that leads to the formation of the corresponding nitrones and hydroxylamines (Scheme 1.4).15 It should be mentioned that very large steric constraints in α-position cause potentially the spontaneous homolytic decomposition of dialkylnitroxides to afford the corresponding nitroso compound and the alkyl radials (Scheme 1.4).16,17
Scheme 1.4 Possible decomposition pathways for nitroxide: (a) fragmentation (b) disproportionation.
However, a few exceptions of stable nitroxides bearing hydrogen atoms on the α-carbons have been reported. For example, due to Bredt's rule we can cite nitroxides containing polycycles (ABNO, AZADO) where the abstraction of the hydrogen in the alpha position atom is forbidden.17 Other examples are related to crowded molecules around the hydrogen to be abstracted or to conformationally constrained nitroxides (TIPNO, SG1, Scheme 1.5) limiting the disproportion reaction to occur.
Scheme 1.5 Examples of stable nitroxides bearing hydrogen atom(s) in the α-position.
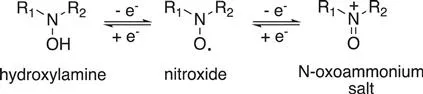
Interestingly, nitroxides behave as redox amphoters (Scheme 1.6). They can be reversibly oxidized to the corresponding N-oxoammonium salt or reduced to the corresponding hydroxylamine in a one-electron process. This property and the tunable redox potentials enable the development of redox probes for biological systems or organic electrodes for lithium batteries or catalytic oxidation reagent in organic synthesis.
Scheme 1.6 Redox properties of nitroxides.
N,N-Dialkyl nitroxides such as TEMPO are usually poorly efficient for C–H activation and H-atom abstraction due to the low OH-bond dissociation energy (BDE, 290 kJ mol−1). Nitroxide bearing electron-withdrawing groups such as α,α-diacyl nitroxides are usually employed for reaction with unactivated positions. For comparison, the BDE of NHPI is much larger (367 kJ mol−1) and the corresponding radical is good H-abstracting reagents. In contrast, the reverse process of converting hydroxylamine to nitroxide is usually thermodynamically favorable and the TEMPO hydroxylamine can be used as an antioxidant.
Therefore, the three-electron bond in nitroxides modifies the usual reactivity of the unpaired electron, notably relative to alkyl or peroxyl radicals, e.g. with no dimerization, limited H-atom abstraction and no reactivity with air and moisture. Moreov...