
- 568 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
This book is a collection of works that canvass many of the recent developments in various areas of connective tissue research. It focuses on the structure of the components, molecular organization and pathology of the extracellular matrix.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Connective Tissue Disease by Jouni Uitto in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Biology. We have over one million books available in our catalogue for you to explore.
Information
I
STRUCTURE AND BIOLOGY OF THE COMPONENTS OF THE EXTRACELLULAR MATRIX
1
The Collagen Family of Proteins
Shriners Hospital for Crippled Children and Oregon Health Sciences University, Portland, Oregon
DOI: 10.1201/9781003210016-2
I. INTRODUCTION
The study of the extracellular matrix is a rapidly expanding field that includes the isolation and characterization of multiple connective tissue macromolecules, the molecular interactions between these connective tissues components, between these components and adjacent cell surfaces, and the effects of these components on the determination and regulation of cellular behavior. Substantial progress has been made in enumerating a variety of glycoprotein and proteoglycan components, some of which are unique to specific types of matrix, and others of which are common to all extracellular matrices. Perhaps because they are the most abundant of these matrix components, the collagens have received a great deal of attention during the past decade (for review, see Refs. 1,2). It is now clear that the term “collagen” represents not a single protein species, but a family of closely related glycoproteins, each of which is genetically distinct, and which has unique structural features and, presumably, a unique function. While considerable direct information has been accumulated regarding the structures of these individual molecules, only indirect information presently exists for their functions.
Since this area of research is in rapid transition, there is considerable confusion concerning identification of these macromolecules, as well as their structure-function relationships in the extracellular matrix. Therefore, it is the purpose of this chapter to review the generally accepted information and to place less emphasis on details presently being disputed. We provide a current catalogue of the various forms of collagen, pointing out similarities and differences, and relate these properties to the ability of these molecules to form specialized fiber systems that have unique functions in the connective tissue matrix. In addition, we briefly summarize the complex intracellular biosynthetic pathways and extracellular processing that leads to the formation of stable fiber forms. It is hoped that this general review will provide a framework for more specific discussion of these events in subsequent chapters.
When the ultrastructure of any connective tissue is examined, a large variety of extra-cellular fibrous structures can be identified. For example, Figure 1 illustrates the ultra-structure of human skin dermal-epidermal junction. The cytoplasm and plasma membranes of the basal cells are adjacent to the connective tissue matrix. In this zone, the basal cells are separated from subjacent matrix by a dense, amorphous structure known as the basement membrane or basal lamina. Subjacent to the basement membrane is the connective tissue matrix. This matrix contains a variety of fibrous structures, the most obvious of which are the large, cross-striated fibers showing a 67-nm periodicity. These are the structures first identified as collagens, and they represent the most common fiber form of these molecules. But, in addition to the banded collagen fibers, a fine, reticular network is also visible. Alfco, in this tissue, banded fibers with a centrosymmetric cross-striation can be seen that appear to originate in the lamina densa of the basement membrane and run perpendicularly from the basement membrane into the upper layers of the dermis. These structures, which are common to basement membrane regions of striated epithelial origin, are known as anchoring fibrils (3). Another fibrous structure can be identified between the hemidesmosomes in the plasma membrane of the basal cells and the lamina densa. These fine filamentous structures have been termed anchoring filaments (4). Many of these fibrous elements are believed to be composed of different collagen types. The lamina densa of the basement membrane is clearly collagenous. The fine filamentous feltwork is an aggregate of at least one other collagen type. There is some evidence that the anchoring fibril is a specialized condensation of yet another collagen type (5). The anchoring filament appears not to be collagenous.
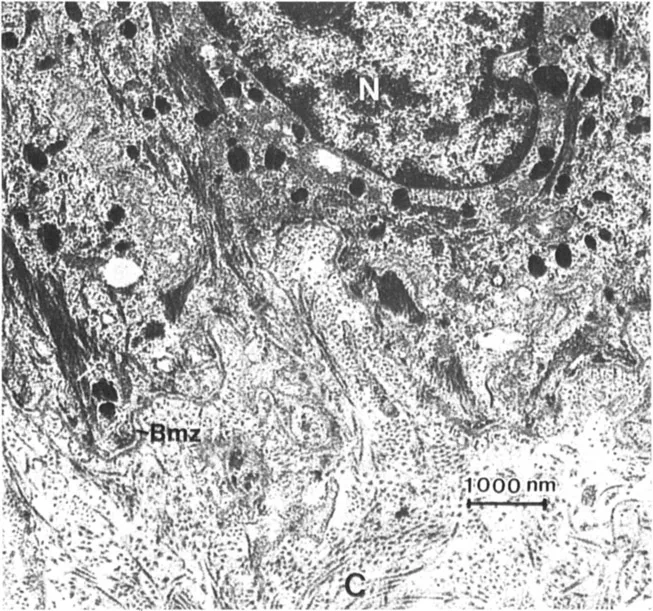
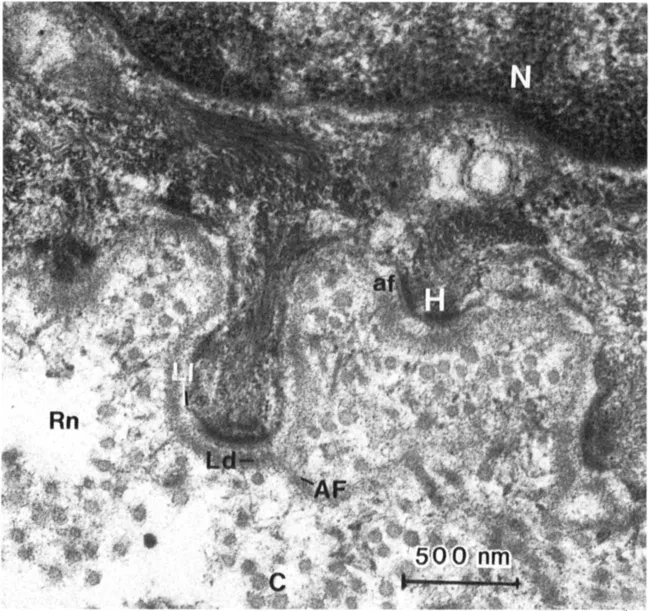
Figure 1 Transmission electron micrographs of the dermal-epidermal junction at two magnifications. A variety of fibrous structure are identified: collagen fibers (C); the basement membrane zone (Bmz), which includes the lamina lucida (LI) and the lamina densa (Ld); anchoring fibrils (AF); anchoring filaments (af); and the reticular network (Rn). The nucleus (N) and hemidesmosomes (H) are also identified.
Figure 1 illustrates several important points, (a) The extracellular matrix is made from a variety of collagen types, (b) The fibrous elements in connective tissues have different fiber forms, and these fiber forms can be attributed to different organizational patterns of the various collagen types, (c) Many of these fiber forms have restricted distributions, such as the basal lamina and the anchoring fibrils.
All these observations suggest that the various members of the collagen family have structural similarities that allow them to form insoluble fiber systems, and each has specific properties that determine unique organizational patterns to serve specific functions which determine and stabilize tissue architecture. This chapter outlines the information contributing to these concepts.
II. THECOLLAGENS
Presently, at least 18 gene products have been identified as subunits of at least 15 collagen molecules. Because of the rapid accumulation of data in this area, the nomenclature of these proteins is confusing and in transition. The most commonly used system for naming these molecules is the use of roman numerals to designate the type of molecule. Therefore, the different collagen molecules are individually indicated as type I, type II, etc. Each collagen molecule contains three polypeptide subunit chains held in a specialized triple-helical array known as the collagen helix. The polypeptide subunits are individually termed α chains. In a given collagen molecule, the α chains may be identical or nonidentical. For example, type I collagen is a heterotrimer of two nonidentical chains. Therefore, these chains are denoted as α1(1) and α2(I). The molecular composition of type I collagen is noted as follows: [α1(I)]2α2(1). In contrast, type II collagen is a homotrimer of three identical chains known as α1(II) chains which form molecules with the composition [α1(II)]3. While this system of nomenclature is extremely useful and specific, it has the inherent difficulty that a newly discovered collagen, which is often initially observed as an individual α chain, cannot be correctly identified as a collagen type and given a roman numeral until the α-chain composition of the parent molecule has been elucidated. For instance, when type III collagen was first identified, the nondenatured molecule (i.e., the native molecule still containing all three polypeptide chains held in triple-helical array) was isolated as a single molecular species and was subsequently denatured into a single type of α chain. In this case, the designation of these a chains as a specific collagen type could be made with relative certainty. In contrast, preparations of native type V collagen isolated from human chorioamniotic membrane released two chains upon denaturation. Therefore, in that tissue several possibilities existed for the molecules containing these chains. They could both be derived from a single heterotrimeric molecule, or each could arise from an independent homotrimeric molecule where the mixture could not be adequately separated under experimental conditions. Therefore, the description of these chains as α1(V) and α2(V) chains could not be made until a molecule with the composition [α1(V)]2α2(V) was identified. To add to the confusion, yet another distinct α chain similar to both the type V chains was identified in other tissues, and the suggestion has been made that it arises from a parent heterotrimeric molecule with the chain composition α1(V)α2(V)α3(V). All these observations suggest that the individual α chains can associate with other chains in a variety of ways to produce different molecular species. This has been strongly suggested for the three chains of type V collagen and for the α1(I) chain, which can associate in the more common heterotrimeric type I molecule or infrequently, into a homotrimeric molecule known as the type I “trimer.” Another limitation of t...
Table of contents
- Cover
- Half Title
- Series Page
- Title Page
- Copyright Page
- Preface
- Contributors
- Contents
- Part I. Structure and Biology of the Components of the Extracellular Matrix
- Part II. Molecular Organization of the Extracellular Matrix
- Part III. Pathology of the Extracellular Matrix
- Index