![]()
1 Angiosperms and Bees: The Evolutionary Bases of Crop Pollination
This volume is about one of the most celebrated relationships between species in all of natural history – that relationship between the bees and the flowering plants, the angiosperms. To be precise, this volume explores the relationship between bees and those angiosperms that make up modern crop plants that depend on bee pollination.
1.1. Sex: Diversity with Stability
For the plants, it is all about sex – that most extravagantly successful (and arguably popular) invention of natural selection that set multicellular organisms on their path toward global dominance. ‘Global dominance?’ you ask, ‘How’s that?’ That’s a fair question when one considers the other successful life alternatives.
The single-celled life alternative is indeed amply represented in Earth’s biota. Just consider the bacteria and archaebacteria that carpet the planet, colonizing virtually every terrestrial and aquatic niche, even penetrating kilometres deep into the planet’s crust. It is these simplest representatives of the biological continuum that baffle us with their boundary bending tolerances to environmental extremes (Merino et al., 2019), making them figure prominently in our discussions about the evolution of life on other planets (Sundarasami et al., 2019).
At the opposite pole of biological organization we have those assemblies of multicellular organisms who have banded together so tightly that we have to consider the group, not the individuals who make it up, as a Darwinian unit of selection. These we call the superorganisms (Wilson and Hölldobler, 2009), most famously represented by the termites, ants, and the social wasps and bees (most wasps and bees are not social), although quirky representatives exist in the forms of a genus of shrimps (Synalpheus spp.) and the naked mole rats of Africa (Heterocephalidae). The ecological impact of the superorganisms is wildly out of proportion to their species count. As one example, the ants and termites make up only 2% of the estimated 900,000 known species of insects on Earth, yet together account for more than half of total insect biomass (Wilson and Kinne, 1990). These are nature’s great recyclers and soil conditioners. Another example is those superorganisms represented by the social bees; these will figure prominently in this volume about bee pollinators of crop plants, although we will also see that their solitary bee cousins are the real workhorses of pollination. To be plain, it is ‘beeness’ that makes a good pollinator, not ‘superorganismness’.
Superorganismality, however fascinating and ecologically important its representatives, is nevertheless a bit of an evolutionary oddball. As far as we can tell, it has independently evolved only 28 times in the history of Earth (Bourke, 2011); all but two of those independent events happening in the insects.
It is the multicellular organisms (hereafter simply ‘organisms’) who occupy the middle of our biological continuum, those bundles of cooperating eukaryotic cells (cells whose DNA is enclosed in a nucleus) who together form a contiguous entity; share a common genetic fate; specialize for the diverse functions of procuring nutrients, defending self and reproducing; and by one means or another resolve internal genetic conflicts. They are the protists, fungi, plants and animals. Together, they are called the Eukarya, one of life’s three domains, or highest taxonomic ranks, standing alongside the Eubacteria and Archaea.
If there is a case to be made that organisms are dominant in the grand scheme of things, it is because they have resolved many of the impediments that hazard the single-celled or superorganismal options. The feverish diversity of body plans and life strategies expressed in organisms have let them approach a measure of the global niche penetration achieved by the more nimble single-celled forms. And owing to the genetic clonality of their body cells, each organism is far more genetically stable than the superorganism, every member of which is an organism in their own right and always poised for mutiny.
Diversity with stability. It is sex that makes all this possible.
Beginning with the eukaryotic single cells and carrying on into the eukaryotic multicellular organism, sex permitted a fresh roll of the genetic dice with every generation, the repeated pairing of unprecedented gene combinations, providing raw fodder for natural selection to act upon. Gene combinations whose phenotypes favoured their transmittal to the next generation were, by logical extension, preserved; unsuccessful combinations were, with symmetrical extension, not. In this way a population’s genes were winnowed and tried against all the extremes its habitat could throw at it. The result was a species optimally adapted to its habitat.
So much for diversity; what about stability?
An emergent outcome of sexual reproduction in organisms is the single-celled zygote, or embryo – that product of the female’s ova fertilized with the male’s sperm. In that one special cell reside all the genetic resources of the future individual. After fertilization and when growth conditions permit, the zygote divides, then divides again, then divides again (1, 2, 4, 8, 16, etc.) in exponential progression until the mature organism is in place. However, the critical point here is that at every division the entire genome is replicated in virtual perfection. The somatic cells of an organism are genetically identical. They are clones and by definition cannot be in conflict.
The sexually derived single-celled zygote is thus the genetic bottleneck that harmonizes genetic variation with clonal compatibility. It is the secret to organisms’ morphological and behavioural diversity, structural complexity, and ecological success among Earth’s biological experiments. It is no accident that it is organisms that come to mind for most of us when we think about life on Earth; it is organisms that Darwin (1859) considered when he wrote On the Origin of Species.
Sex is a big deal then, and it was taking place at the very beginning for the angiosperms and plants in general.
1.2. Sex in the Gymnosperms
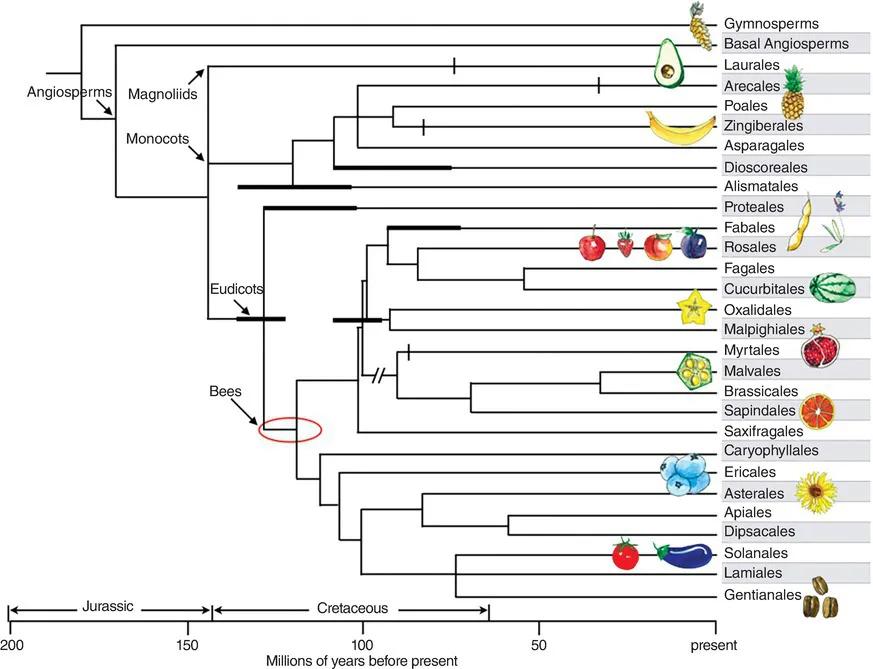
Rather than begin with primitive plants, let us jump to the gymnosperms, the nearest older relatives to the angiosperms (Fig. 1.1). Gymnosperm ovules are ‘naked’ (hence the Greek name gymnos) and remain exposed on the surface of leaf-derived structures called bracts, which when tightly concentrated together are called cones. The sexual structures are segregated into male cones and female cones. Pollen is transferred from male to female cones by abiotic vectors such as water and wind, the first pollinating agents (Ollerton and Coulthard, 2009). The morphology of windborne pollen reflects its mode of transfer by wind. Under magnification, windborne pollen grains appear dry, smooth and small to moderate in size; moreover, the pollen is produced in huge quantities (Ackerman, 2000). Anyone who lives in pine regions where windborne pollen blankets the landscape every spring, can appreciate the vast scales in quantity and space possible with gymnosperm pollination. However impressive these seasonal surges, from a biological point of view they are indiscriminate in pollen’s spread and deposition, profligate in their wastage of it, and ultimately limited in the efficiency by which they ensure plant mating, reproduction, and range expansion.
Fig. 1.1. Phylogeny showing chronology of angiosperm divergence and position of orders containing the major bee-pollinated crop plants listed in Table 3.1. Adapted from topology of Byng et al., 2016, superimposed with geological divergence dates of Bell et al., 2010. Gymnosperms are supported as a monophyletic sister group to the angiosperms from Bowe et al., 2000. Bold lines indicate where topology is sustained with the confidence intervals of Bell et al., 2010. Vertical tick marks indicate divergence chronology for the crown taxon. Divergence dates for bees from Cardinal and Danforth, 2013. Icons show representative crop members of each order.
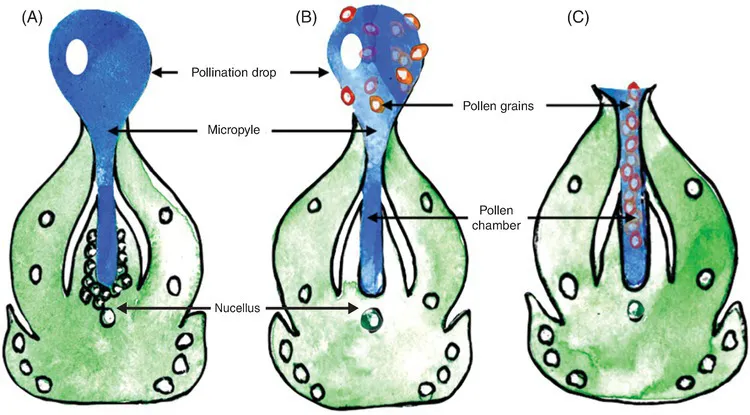
Among the surfaces indiscriminately dusted with pine pollen are female cones and their exposed ovules. Each ovule excretes a solution called a pollination drop that extends beyond the terminus of the micropyle – a small opening at the apex of each ovule. (Fig. 1.2). This pollination drop serves as a landing site for airborne pollen. Once pollen lands on it, the drop recedes back into the interior of the ovule, carrying the pollen with it, facilitating pollination and subsequent fertilization and maturation of the seed.
Fig. 1.2. Gymnosperm pollination and fertilization. The pollination drop in gymnosperms is a precursor to angiosperm nectar. The sugary solution extends beyond the micropylar opening of an ovule (A). After airborne pollen lands on it (B), the droplet recedes back into the pollen chamber (C), facilitating pollination. The pollination drop is secreted by cells in the nucellus – ovular tissues that contain the embryo sac. Redrawn from Jin et al., 2012.
1.3. Flower Morphology and Fertilization
Now for some terms.
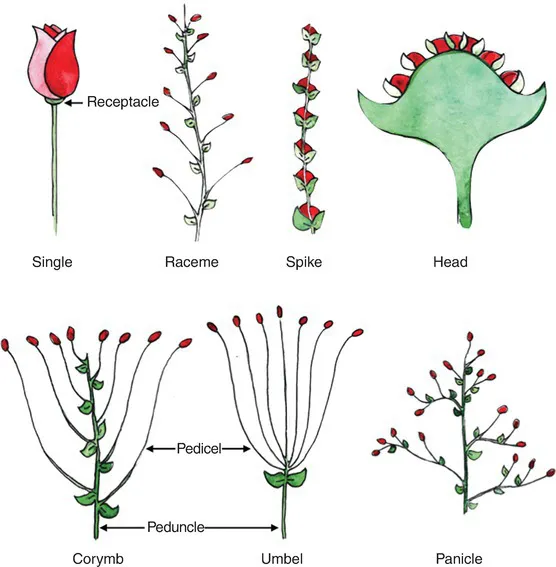
A flower is a plant organ unique to the angiosperms, evolved for increasing efficiency of sexual reproduction. An inflorescence is an arrangement of flowers on a stem. The main stem of an inflorescence is the peduncle, and the stem of any individual flower is the pedicel. The thickened end of the pedicel forming the base of the flower is the receptacle. The configuration of inflorescences are variations on the presence, arrangement and point of origin of pedicels relative to the peduncle; a sampling of their multiplicity of form is shown in Fig. 1.3. A raceme has a series of unbranching pedicels along a central axis and no terminal flower. A spikeis a kind of raceme without pedicels. A head, also called a composite flower, can be thought of as a concentrated raceme in which individual flowers are massed together onto one enlarged receptacle. A corymb is flat topped or convex, with long proximal pedicels becoming increasingly shorter as they move distally, and lacking a terminal flower. An umbel resembles a corymb, but all pedicels are of equal length and originate from one point of attachment. A panicle, or compound raceme, is irregularly branched with each branch possessing a terminal flower.
Fig. 1.3. Some examples of inflorescence designs. The floret in each example is indicated in red.
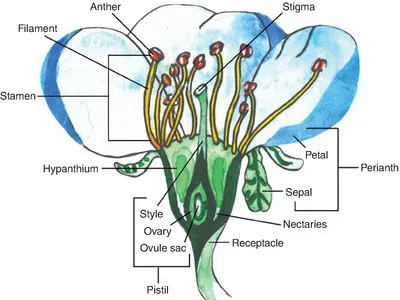
A flower’s outer whorl of petals is called the corolla and functions to protect the interior sexual parts, to exclude ineffective pollinators, to attract effective pollinators, and to direct effective pollinators toward the inside of the flower (Fig. 1.4). In legume-type flowers, two anterior petals are modified to form a keel inside which are housed the sexual parts of the flower. At the base of the corolla are the calyx or sepals; typically green and leaf-like, sepals protect the flower in bud and provide structural support in bloom. Collectively, the non-sexual parts of the flower – the sepals and petals – are called the perianth. In some of the basal angiosperms it is difficult to distinguish sepals from petals, in which case the structures are called tepals (Endress, 2008). The aggregate structure comprising the bases of the sepals, corolla and stamens is called the hypanthium and often contains the nectaries.
Fig. 1.4. General morphology of a hermaphrodite (also called perfect or bisexual) flower. The pistil (female part of the flower) in this case has only one carpel and is an example of a perigynous ovary (see Fig. 3.2, this volume).
Male parts of a flower are called the stamens, each made up of a slender filament holding an anther at the tip. When it is mature, the anther opens and exposes or releases pollen grains which contain the equivalent of animal sperm. I...