
Drug Delivery Approaches
Perspectives from Pharmacokinetics and Pharmacodynamics
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Drug Delivery Approaches
Perspectives from Pharmacokinetics and Pharmacodynamics
About this book
Explore this comprehensive discussion of the application of physiologically- and physicochemical-based models to guide drug delivery edited by leading experts in the field
Drug Delivery Approaches: Perspectives from Pharmacokinetics and Pharmacodynamics delivers a thorough discussion of drug delivery options to achieve target profiles and approaches as defined by physical and pharmacokinetic models. The book offers an overview of drug absorption and physiological models, chapters on oral delivery routes with a focus on both PBPK and multiple dosage form options. It also provides an explanation of the pharmacokinetics of the formulation of drugs delivered by systemic transdermal routes.
The distinguished editors have included practical and accessible resources that address the biological and delivery approaches to pulmonary and mucosal delivery of drugs. Emergency care settings are also described, with explorations of the relationship between parenteral infusion profiles and PK/PD. The future of drug delivery is addressed via discussions of virtual experiments to elucidate mechanisms and approaches to drug delivery and personalized medicine.
Readers will also benefit from the inclusion of:
- A thorough introduction to the utility of mathematical models in drug development and delivery
- An exploration of the techniques and applications of physiologically based models to drug delivery
- Discussions of oral delivery and pharmacokinetic models and oral site-directed delivery
- A review of integrated transdermal delivery and pharmacokinetics in development
- An examination of virtual experiment methods for integrating pharmacokinetic, pharmacodynamic, and drug delivery mechanisms
- Alternative endpoints to pharmacokinetics for topical delivery
Perfect for researchers, industrial scientists, graduate students, and postdoctoral students in the area of pharmaceutical science and engineering, Drug Delivery Approaches: Perspectives from Pharmacokinetics and Pharmacodynamics will also earn a place in the libraries of formulators, pharmacokineticists, and clinical pharmacologists.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1
Introduction: Utility of Mathematical Models in Drug Development and Delivery
1.1 Introduction
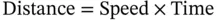
1.2 Use of Mathematical Models in Drug Development
Table of contents
- Cover
- Table of Contents
- Title Page
- Copyright
- Preface
- 1 Introduction: Utility of Mathematical Models in Drug Development and Delivery
- 2 Physiologically Based Models: Techniques and Applications to Drug Delivery
- 3 Oral Delivery and Pharmacokinetic Models
- 4 Oral Site‐Directed Drug Delivery and Influence on PK
- 5 The Vasoconstrictor Assay (VCA): Then and Now
- 6 Topical Delivery: Toward an IVIVC
- 7 Integrated Transdermal Drug Delivery and Pharmacokinetics in Development
- 8 Formulation and Pharmacokinetic Challenges Associated with Targeted Pulmonary Drug Delivery
- 9 Oral Transmucosal Drug Delivery
- 10 PK/PD and the Drug Delivery Regimen for Infusion in the Critical Care Setting
- 11 Virtual Experiment Methods for Integrating Pharmacokinetic, Pharmacodynamic, and Drug Delivery Mechanisms: Demonstrating Feasibility for Acetaminophen Hepatotoxicity
- 12 Personalized Medicine: Drug Delivery and Pharmacokinetics
- Index
- End User License Agreement