
- 426 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Engineering Catalysis
About this book
The book illuminates various aspects of heterogeneous catalysis engineering, from catalysis design, catalyst preparation and characterization, reaction kinetics, mass transfer, and catalytic reactors to the implementation of catalysts in chemical technology. Aimed at graduate students, it is also a useful resource for professionals working in research and development.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1 The basics
1.1 Catalytic concepts
1.1.1 Definitions
Catalysis is a phenomenon related to the acceleration of rates of chemical reactions.
Such acceleration was recognized long time ago and put into practice even before the concept of catalysts was formulated. For example, a German chemist Döbereiner discovered that oxidation of hydrogen can occur in the presence of Pt already in 1823. Such invention resulted in substantial utilization of Döbereiner lamps just within few years after the discovery.
The available data on the conversion of starch to sugars in the presence of acids, combustion of hydrogen over platinum, decomposition of hydrogen peroxide in alkaline and water solutions in the presence of metals, were summarized by a Swedish scientist J. J. Berzelius in 1836 [1], who proposed the existence of a certain body, which “effecting the (chemical) changes does not take part in the reaction and remains unaltered through the reaction”.
Catalytic power or force, according to Berzelius, meant that “substances (catalysts) are able to awaken affinities which are asleep at this temperature by their mere presence and not by their own affinity”.
This concept was immediately criticized by Liebig, as this theory was placing catalysis somewhat outside of other chemical disciplines [2]. A catalyst was later defined by Ostwald as “a compound that increases the rate of a chemical reaction, but which is not consumed by the reaction” [3]. This definition allows for the possibility that small amounts of the catalyst are lost in the reaction or that the catalytic activity slowly declines.
From these definitions, a direct link between chemical kinetics and catalysis is apparently clear as, accordingly, catalysis is a kinetic process. There are, however, many issues in catalysis that are not directly related to kinetics, such as mechanisms of catalytic reactions, elementary reactions, surface reactivity, adsorption of reactants on solid surfaces, synthesis and structure of solid materials and catalytic engineering.
An important issue in catalysis is selectivity towards a particular reaction. For example, transformation of synthesis gas (a mixture of CO and hydrogen) can lead either to methanol (on copper) or high alkanes (on cobalt). For consecutive reactions it could be desirable to obtain an intermediate product. In the oxidation of ethylene such target is ethylene oxide, but not CO2 and water.
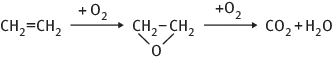
Although formaly catalysts remained unchanged during a reaction, they are involved in chemical bonding with the reactants during the catalytic process in a cyclic process: the reactants are bound to one form of the catalyst, and the products are released from another, regenerating the initial state (Fig. 1.1).
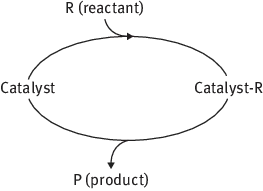
Fig. 1.1: Catalytic cycle.
It should be also noted that although it is usually assumed that a catalyst participates in the process but remains unchanged at the end, there could be major changes in its structure and composition.
Potential energy diagrams for the catalytic and non-catalytic reactions presented in Fig. 1.2 indicate that in both cases the reactions should overcome a certain barrier, which is lower in the presence of a catalyst.
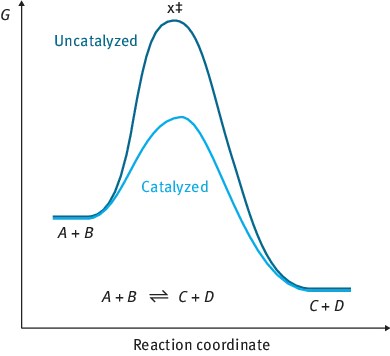
Fig. 1.2: Potential energy diagram for catalytic and non-catalytic reactions. Changes of Gibbs energy along the reaction coordinate.
As follows from Fig. 1.2, the change in the Gibbs free energy between the reactants and the products ΔG is the same, independent of the presence or absence of a catalyst, but providing, however, an alternative reaction path.
A lower value of activation energy implies higher reaction rates, which could be expressed through the rate constant k dependence on temperature:
(1.1)
where A is the pre-exponential factor, Ea is the activation energy related to the potential energy barrier and m is a constant. Equation (1.1) was proposed by Kooij and van’t Hoff [4] to explain the temperature dependence of reaction rates and could be derived from the transition state theory of Eyring and Polanyi [5]. Arrhenius [6] applied a slightly simplified form:
(1.2)
A decrease in activation energy and an increase in temperature lead to an increase of the rate constant and thus of the reaction rate. Catalysts substantially accelerate chemical reactions, enabling them to be carried out under the most favorable thermodynamic regime, and at much lower temperatures and pressures.
Equation (1.2) corresponds to an elementary reaction. In the case of complex reactions, it is appropriate to discuss the so-called apparent activation energy, which can be expressed through the reaction rate r according to
(1.3)
Table of contents
- Title Page
- Copyright
- Contents
- Preface to the first edition
- 1 The basics
- 2 Engineering catalysts
- 3 Engineering reactions
- 4 Engineering technology
- Acknowledgments
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Engineering Catalysis by Dmitry Yu. Murzin in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemistry. We have over one million books available in our catalogue for you to explore.