Influenza Prophylaxis and Treatment
Irina Kiseleva, Natalie Larionova Abstract
Each year, influenza causes a significant acute respiratory disease burden. Also, influenza pandemics periodically occur. Annual vaccination is the best tool for influenza prevention, but its effectiveness can vary from year to year. Antiviral treatment shortens the clinical course if administered within the first hours of the onset of the disease. Neuraminidase inhibitors (oseltamivir, zanamivir, and peramivir) and polymerase inhibitors (baloxavir) are currently used as antivirals. Limited specificity of conventional vaccines and drug resistance of circulating viruses reduce the effectiveness of prophylaxis and treatment, requiring the development of new broad-spectrum drugs.
Keywords: Cross-protectivity, Direct-acting antivirals, Drug resistance, Inactivated influenza vaccines, Indirectly acting antivirals, Live attenuated influenza vaccines, Next-generation vaccines, Synthetic vaccines.
INTRODUCTION
In the first quarter of the 21st century, influenza epidemics and pandemics continue to be a threat to humankind. Of course, seasonal influenza remains one of the major public health threats. However, concerns regarding the possibility of pandemic spread of novel influenza strains have led to increased interest in new influenza vaccines and drug development.
Isolation of the first influenza virus led to the development of the first generation of inactivated and live influenza vaccines. A hundred years after the influenza virus was first isolated, effective influenza vaccines are still an important influenza prevention tool and are the key element in influenza control [1]. Vaccination can reduce illness and lessen the severity of infection, especially in high-risk groups [2]. Innovative strategies, such as recombinant technologies, are currently being studied to improve the immunological response to vaccination [3] and develop universal vaccines [4].
The current strategy for the fight against influenza infection involves the development of a global epidemiological surveillance network [5], methods of rapid diagnosis, preventive vaccination of the population and the use of antivirals
[6]. The recommendations of the European Center for Disease Control emphasize that vaccination is the main, most effective method of preventing the severe and complicated course of influenza infection, even if the vaccine is less effective than expected [7].
Disease management through antivirals is also an important component of the current intervention strategy to prevent and treat this infection. Antiviral treatment shortens the clinical course when given within two days from the onset of the disease. Antiviral drugs have been approved for treatment or prophylaxis of influenza A and B, caused by circulating influenza virus strains [8]. They are necessary in cases of diseases of non-immunized persons, and when the composition of the vaccine strains does not correspond to the viruses that caused the epidemic/pandemic.
Approved antiviral drugs for influenza fall into three classes, M2 channel inhibitors, neuraminidase inhibitors, and polymerase inhibitors [9-11]. Unfortunately, drug resistance of circulating viruses reduces the effectiveness of influenza treatment with approved antivirals and dictates the need for the development of new influenza antivirals.
PAST, PRESENT, AND FUTURE OF INFLUENZA VACCINES
Influenza vaccines may be classified into two basic types, namely live attenuated (LAIV) and inactivated (IIV) vaccines. LAIV can replicate in the susceptible hosts and stimulate an immune response by mimicking natural disease. The inactivated vaccine candidate contains the whole virus or parts of it; it is killed by special treatment and cannot replicate. Unlike LAIV, IIV typically requires multiple doses to ensure a sufficient immune response.
Besides, several subtypes of influenza vaccines exist. All of them will be discussed below in other sections.
Substrates for Influenza Vaccine Development
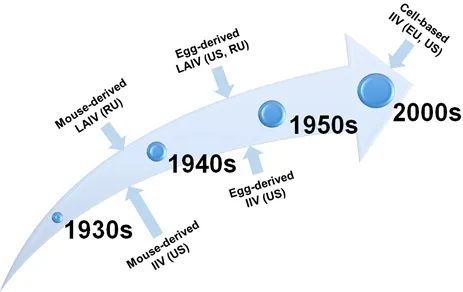
In the 1930s, the first influenza viruses were isolated in ferrets [12] and mice [13-15]. In 1938, a Russian live attenuated vaccine was developed by Smorodintsev in mice which were the most employed animals in virology [14]. This vaccine was developed by passing of the influenza virus through mice based on a genius Louis Pasteur's method of attenuation by sequential passing of the pathogen in another host. This mouse-derived LAIV was administered intranasally and used for influenza prophylaxis in adults in the USSR until 1947 (Fig. 1). The following Fig. (1) displays the most important milestones in the development of influenza vaccines.
During the same period, another mice-derived influenza vaccine was developed in the US. It was a vaccine obtained directly from mouse lungs, or the mouse-derived virus was introduced into the so-called “tissue culture medium” which consisted of a minced chicken embryo [16]. Like the intranasal Russian vaccine, this preparation also contained live virus; however, it was administered subcutaneously and intradermally, and therefore can be considered as the first prototype of the inactivated influenza vaccine (IIV).
When chicken embryos were discovered as an alternative system for isolation of different viruses [17], a new era of virus isolation, including the influenza virus, began. After 1947, mice as the substrate for Russian LAIV development were completely replaced with embryonated chicken eggs (herein simply referred to as “eggs”). The US IIV and LAIV technology processes were also employed for egg-based vaccine production.
Fig. (1)) Schematic evolution of influenza vaccine strategy based on the substrate for cultivation of influenza virus.
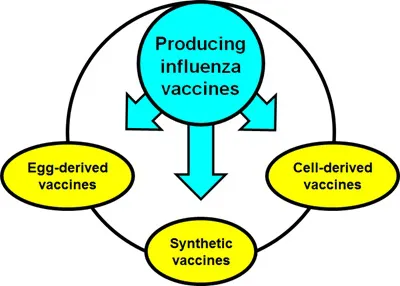
Today, three parallel approaches for influenza vaccine production are being used by vaccine manufacturers: (i) the production of conventional influenza vaccine in eggs, (ii) the production of the conventional influenza vaccine in cell culture, and the most recent technology is (iii) the production of so-called synthetic vaccines (Fig. 2).
Fig. (2)) Conventional and novel platforms for influenza vaccine production.
Conventional Egg-Derived Influenza Vaccines
The majority of influenza vaccines that are currently commercialized by licensed manufacturers are grown in eggs. “This is a labor-intensive process as each egg must be sterilized, candled, inoculated with the virus, and incubated before harvesting small volumes of allantoic fluid from each egg and pooling before purification” [18]. For each dose of inactivated vaccine, at least 1-2 eggs are required. For 10-15 doses of a live vaccine, the same number of eggs (1-2) are required. Therefore, millions of embryonated chicken eggs are required each year for seasonal vaccine production. In the event of a pandemic situation, their number will progressively increase.
Moreover, for a long time, eggs were the only WHO-approved substrate for influenza virus isolation, despite that in the 1990s, it was already shown that egg-derived variants ...