There are a large range of microporous materials and these can have a variety of chemical compositions, whether inorganic or organic in nature, as well as hybrid materials containing both inorganic and organic components. Microporous materials also vary by their degree of crystallinity, with some having both short- and long-range order, and amorphous porous materials lacking any long-range order at all. In this section, the key classes of porous material that are discussed in later chapters will be introduced.
1.2.1 Zeolites
Zeolites are naturally occurring crystalline aluminosilicate minerals that are typically found in regions where there has been volcanic activity. The name zeolite originates from how the rock appeared to ‘boil’ upon heating, with the ancient Greek zein for ‘to boil’ and lithus for ‘rock’, so literally ‘zeolite’ means ‘boiling rock’. It was not until the 1940s that Barrer found a method to synthesise zeolites in a laboratory. Barrer was able to synthesise zeolites via mimicking the conditions where they form naturally, in particular the high temperature and pressure, along with organic template molecules that would direct the formation to a targeted zeolite structure. There are now more than two hundred zeolite structures that are either naturally occurring or have been synthesised in the laboratory.1
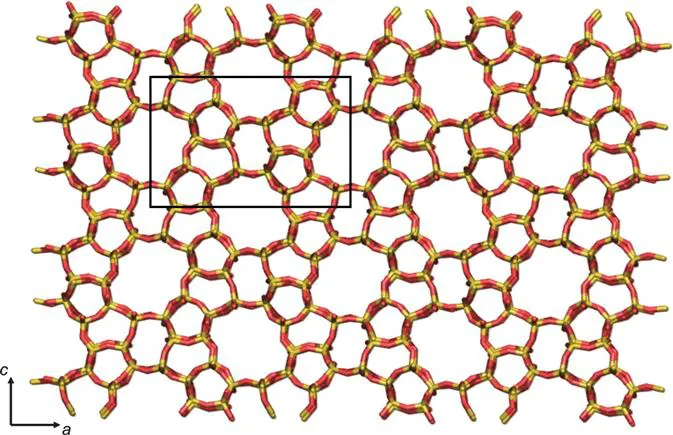
Zeolites are composed of [SiO4]4 − and [AlO4]5 − tetrahedra, and these primary building units, known as T-sites, are linked by the bridging oxygen atoms to produce corner-sharing tetrahedra. Through the multitude of ways that these tetrahedra can be linked and arranged to form crystalline arrays, there are millions of hypothetical zeolite topologies. There are a series of what are known as ‘secondary building units’ or SBUs that can be used to characterise a zeolite topology. Examples of SBUs include n-membered rings (where n = 3, 4, 5, 6…), and then the equivalent doubled rings, double 4-ring (D4R), double 5-ring (D5R), double 6-ring (D6R) and so on that have n bridging oxygens linking the two rings, as well as a small number of other SBUs linking different sized rings. Each zeolite has a three letter framework type code assigned by the International Zeolite Association,1 which will be unique to a zeolite topology. An example of the zeolite with the framework type code MFI is shown in Figure 1.1.
Figure 1.1 An example of a zeolite structure. This is the zeolite MFI viewed down the b-axis, with the a- and c-axes of the unit cell (shown in black) labelled. Ten membered-ring channels run down the a- and b-axes.
The microporosity of zeolites originates either from the channel formed in an SBU or from the channels formed between SBUs when they are connected into a zeolite topology. The pore dimensions of zeolites vary across the full range of the definition of microporous materials. The first SBU where a small guest can feasibly pass through is the 6MR, with a diameter of ∼2 Å. Not only will the pore dimensions of different zeolite structures vary, but obviously also the shape of the channels, such as straight or curved channels, and some with larger cavities at points in a channel or side pockets connected to a main channel. Zeolites vary further by their pore dimensionality. There are zeolites with only 1-dimensional channels, those with 2-dimensional channels and those with 3-dimensional interconnected channels.
In terms of chemical composition, while an all-siliceous zeolite would only contain [SiO4]4 − tetrahedra, typically zeolites are aluminosilicates, with some [SiO4]4 − substituted for [AlO4]5 − tetrahedra. While the chemical composition may vary, all zeolites with the same framework structure (topology) will still have the same framework type code. For example, the code MFI refers to the structure that is common to both the all-siliceous silicialite-1 material (see Figure 1.1) and for ZSM-5 that contains both silicon and aluminium. In every instance where an Al3 + ion substitutes a Si4 + ion, there needs to be a ‘charge-compensating’ cation to maintain the zeolite structure's overall charge neutrality. These cations are generally not part of the framework itself but are more loosely bound in the zeolite's pores. The cations can be the organic templates used in the synthesis, or metal cations such as sodium or calcium, with the latter ions generally being solvated by water unless the zeolite has been dehydrated. Alternatively, the compensating cation can be a proton, and this then imparts a high acidity to the zeolite structure, that can be used in catalytic applications. Overall, the majority of zeolite applications stem from the presence of compensating cations and thus as a result of the aluminium substitution in the framework. The Si/Al ratio for a zeolite can range from 1 to infinity, with the lower limit of 1 a result of Lowenstein's rule that states that [AlO4]5 − tetrahedra will not form direct Al–O–Al bridges.
Beyond zeolites, there are a range of related materials known as ‘zeotypes’. Zeotypes can adopt the same framework structures as zeolites, but have different chemical compositions. For example, there is the family of aluminophosphates (ALPOs) consisting of Al3 + and P5 + ions (without the need for any compensating cation), SAPOs consisting of silicon, aluminium and phosphorus, zinc phosphates, and germanium sulfides. Through altering the chemical composition and in particular through the possibility to incorporate transition metal ions, this brings access to a further range of properties, particularly in terms of catalysis.
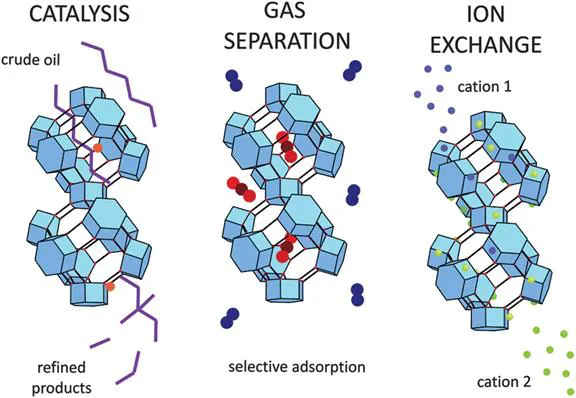
The applications for zeolites stem in particular from their chemical and physical stability, the presence of charge compensating cations, and the fact that zeolite pore dimensions are commensurate with small guest molecules and zeolite structures are available with a wide range of pore dimensions, shapes and topologies, allowing one to ‘pick’ an appropriate zeolite for a specific target application. Naturally occurring zeolites are typically very cheap and available on a large scale, and some synthetic zeolites are also available at a reasonably low cost. Zeolites have three major applications, several of which involve multimillion tonne productions. These applications are in ion exchange, catalysis and as molecular sieves (see Figure 1.2).
Figure 1.2 The main applications of zeolites.Reproduced from ref. 2 with permission from the Royal Society of Chemistry.
In ion exchange, the charge compensating cations in the zeolite are able to be exchanged with other cations, as the cations remain loosely bound. The selectivity of a zeolite towards a cation is determined by the topology and chemical composition, as this will affect the ability of an ion to diffuse through the pores and the strength of the binding at the sorption site. Zeolite A has been used in ion exchange on a large scale in washing detergents, where it acts as a water softener by selectively removing Ca2 + ions from hard water. Zeolites have also been used to remove radioactive ions from nuclear wastes or after nuclear disasters, or toxic ions from the waste water of heavy industry.
Zeolites are highly hydrophilic materials and will absorb significant quantities of water even at raised temperatures. This has led to their use as drying agents, for example, in hospitals for drying gases used for medical treatments or on a large scale in chemical industry, such as drying liquid propane. Zeolites can perform molecular separations, thus acting as ‘molecular sieves’, by several different mechanisms:
- (i) Size exclusion: excluding molecules with dimensions larger than the zeolite's pores, and thus separating the larger molecules from a mixture.
- (ii) Sorption differences: where two different components of a mixture have different enthalpies of sorption within a zeolite. For example, in pressure swing adsorption, a mixture would be forced into a zeolite under pressure, then the pressure will be lowered slightly and only the more weakly bound guest component will be released from the z...