Key Concepts
The GI mucosa is the major immunologic site of contact between the body and the external world.
The manner in which immune cells encounter antigen determines the subsequent immunologic response.
Oral tolerance is a complicated process, probably proceeding by several overlapping mechanisms.
Many factors, including developmental stage, microbial exposures, diet and genetics, influence the balance between allergy and tolerance.
Introduction
The mucosa is the principal site for the immune system’s interaction with the outside environment. Unlike the skin, which is characterized by many layers of stratified epithelium, the intestinal mucosa is lined with a single layer of columnar epithelium. Almost two tons of food travel past this thin barrier each year. More than one trillion bacteria representing about 500 distinct species live in contact with it. The vast majority of these bacteria are non-pathogenic commensals, but pathogens lurk in this diverse antigenic stew, and even the commensal bacteria have the potential to cause harm if not kept in check. The mucosal immune system performs the essential job of policing this boundary and distinguishing friend from foe.
Not only must the mucosal immune system determine the local response to an antigen, but, as the primary site of antigenic contact for the body, it also plays a central role in directing the systemic response to antigens. Oral tolerance – the modulation of the immune response to orally administered antigens – is a fundamental task of the mucosal immune system. In general, as befits the ratio of benign to pathogenic antigens it encounters, the default response of the mucosal immune system is tolerance. The tendency to tolerize to fed antigen can even be used to overcome already developed systemic sensitization, something known and exploited long before the specific cells comprising the immune system were identified. Yet, despite the general bias toward tolerance, the mucosal immune system is capable of producing protective responses to pathogens. This response is controlled by recognition of inherent characteristics of the antigen, or contextual cues such as tissue damage. In general, the immune system is remarkably skilled at responding properly to the antigens it encounters. Failures, albeit uncommon, can be very serious. Food allergy is a prime example of the failure of oral tolerance.
How the mucosal immune system determines when to sound the alarm and when to remain silent is the focus of this chapter. In it, we examine the normal response to food proteins, how that response can go awry, and the factors that tip the balance.
Structure and function
The primary role of the GI tract is to absorb food and liquid and eliminate waste. To achieve this goal, the surface of the tract is both enormous (100 m2) and extremely thin. The lumen of the intestinal tract provides a hospitable environment for bacteria that help break down foods into absorbable nutrients. However, the thinness of the barrier between external and internal creates a grave danger. It is not just nutrients, but toxins, pathogenic bacteria, viruses and parasites that are kept out by a single cell layer only. Breaks in this thin barrier create a risk of systemic infection. The complex task of protecting this border involves both non-specific and highly targeted techniques.
Chemical defenses
Protection begins with chemical and physical measures that keep some of the potentially harmful antigens (both food and microbial) from contact with the mucosal immune system and thus from generating an inflammatory response. Although the intestinal lumen is one of the most microbiologically dense environments in the world, bacteria and large antigens are actually maintained at some distance from the epithelial cells that line the GI tract. This is accomplished by a rich glycocalyx mucin layer (the mucus), which is produced by specialized intestinal epithelial cells. Antimicrobial peptides are caught in the mucous layer in a concentration gradient that provides a zone of relative sterility immediately proximal to the epithelial layer. In mouse models, deficiency of either the mucins or the antimicrobial peptides results in chronic inflammation. In humans, mutations causing abnormal production of the antimicrobial peptides are associated with the autoimmune syndrome Crohn’s disease.1,2 Whether dysfunction in the mucous layer or antimicrobial peptides play a role in the development of food allergy is an area yet to be explored.
What is known is that the enzymatic degradation of food proteins is a first line of protection against allergic sensitization, and that defects in digestion of food antigens contribute to allergy. Many food proteins never get a chance to cause the systemic immune responses characteristic of allergy because they are labile and are denatured by the acidic contents of the stomach. Allergens tend to be proteins that are resistant to this degradation, and thus capable of reaching immune cells to cause sensitization and reaction. For example, β-lactoglobulin and Ara h2, some of the relevant allergens for milk and peanut allergy, respectively, are not denatured by the conditions of the GI tract. Other potential allergens, such as the birch homologs found in many fruits, are easily broken down: although they can induce oral symptoms in cross-reactive individuals, they do not typically initiate sensitization by themselves. Several studies have lent evidence to the importance of the normal enzymatic processes in preventing allergy by showing that antacids impair oral tolerance in both animals and humans. Further, in mice, encapsulation of potentially allergenic foods facilitated allergy by allowing intact allergen to be present in the small intestine.3
The fact that most proteins are broken down by acid and enzymes may help explain why most foods tend not to be allergens, but it does not explain why allergy to stable proteins remains relatively rare. Peanut, for example, contains several proteins that are not degraded, yet only about 1% of the US population is allergic to it, despite near universal exposure. Clearly, other factors come into play after the digestive processes of the stomach.
Trafficking of antigen across the epithelium
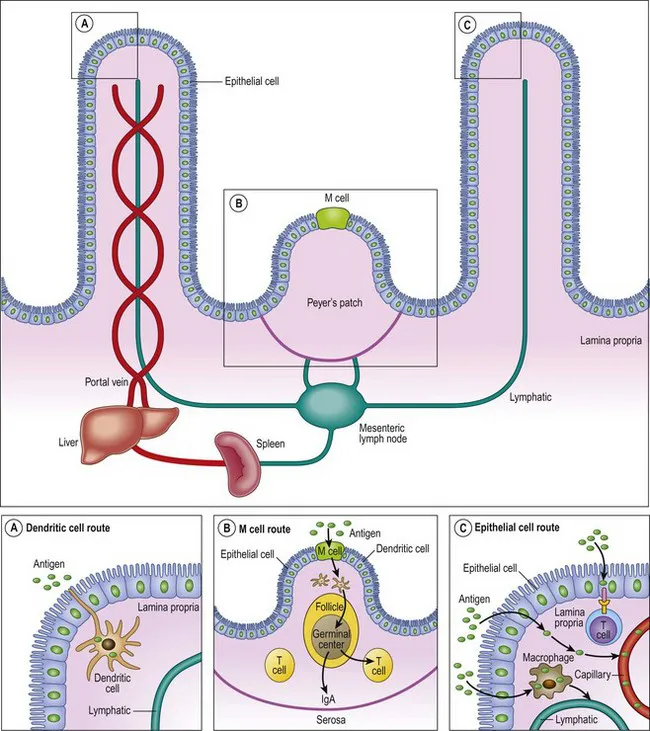
Proteins that are not degraded by enzymatic processes can come into contact with the immune system in a number of ways. Transport across the epithelium is both active and passive, occurring both in the spaces between the cells and across them (Fig. 1.1).
The high-volume route for fluid is via the paracellular spaces, and the overall permeability of the mucosa is regulated by tight junctions that seal the space between epithelial cells. The leakiness of these junctions is subject to a variety of factors, including cytokines, medications and nutritional status. Permeability varies along the GI tract, and even within a short area, as the pores of the villi allow passage of larger solutes than those of the crypt.2,4 Cytokines associated with both autoimmune and allergic disease disrupt barrier function and increase permeability.5 Children with food allergy have been shown to have increased intestinal permeability, both at a time when they are regularly consuming the relevant allergen and after a long period of avoidance.6,7 Other evidence for the importance of barrier function in allergy is the high rate of new sensitization in people taking the anti-rejection medicine tacrolimus, which causes mucosal barrier dysfunction. Although tacrolimus has other effects on the immune system, the high rate of new food allergies after solid organ transplantation is thought to be due its effects on mucosal integrity.5
In addition to the paracellular route, several alternative transport systems actively carry proteins, electrolytes, fatty acids and sugars across cells. Specialized modified epithelial cells called M (or microfold) cells act as non-professional antigen-presenting cells. These cells stud the follicle-associated epithelium overlying specialized collections of immune cells called Peyer’s patches. They express receptors that recognize microbial patterns and aid in the endocytosis and transfer of antigen to the basal surface of the epithelium. This is especially important for bacteria, but may also be relevant for food allergens.4
Other non-specialized columnar epithelial cells form vesicle-like structures that allow transport of dietary proteins across cells. The formation of these vesicle-like structures seems to be dependent on MHC class II binding, but transocytosis can also occur via binding of antigen to IgA, IgE, and IgG. Transport via IgE may be especially important in the acute allergic response and in the amplification of allergy.4 In contrast, secretory IgA, which accounts for the majority of the immunoglobulin produced by the body, complexes with antigen and facilitates transport across the epithelium to antigen-presenting cells, with a tolerogenic outcome.
A final method of antigen transport...