
eBook - ePub
Clinical Gynecologic Oncology E-Book
Philip J. DiSaia, William T. Creasman, Robert S Mannel, D. Scott McMeekin, David G Mutch
This is a test
Share book
- 700 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Clinical Gynecologic Oncology E-Book
Philip J. DiSaia, William T. Creasman, Robert S Mannel, D. Scott McMeekin, David G Mutch
Book details
Book preview
Table of contents
Citations
About This Book
The most readable, most comprehensive book in its field, Clinical Gynecologic Oncology, 9th Edition is the leading reference for diagnosis and treatment of gynecologic cancers – a must-have reference for improving outcomes and providing effective care. A "who's who" list of contributing authors, under the editorial direction of Drs. Philip DiSaia and William Creasman, provides expert guidance on clinical presentations and management, now fully up to date with a brand-new design for faster, easier reference.
- Contains useful appendices covering staging, screening, nutritional therapy, toxicity criteria, blood component therapy, and radiation therapy.
- Covers hot topics such as multi-panel genetic testing, target therapies, sentinel node concept in endometrial cancer and vulvar cancer, and robotic surgery.
- Updates include new quick-reference features such as key point boxes with bulleted lists, highlighted key text, enhanced chapter outlines, and a brand-new design throughout.
- Includes up-to-date references and algorithms, making this text a comprehensive resource for clinical practice, personal study, and exam review.
- Helps you take advantage of the latest advances in early detection and improved treatment options for gynecologic cancers, especially uterine and cervical cancers.
Frequently asked questions
How do I cancel my subscription?
Can/how do I download books?
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
What is the difference between the pricing plans?
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
What is Perlego?
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Do you support text-to-speech?
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Is Clinical Gynecologic Oncology E-Book an online PDF/ePUB?
Yes, you can access Clinical Gynecologic Oncology E-Book by Philip J. DiSaia, William T. Creasman, Robert S Mannel, D. Scott McMeekin, David G Mutch in PDF and/or ePUB format, as well as other popular books in Medizin & Gynäkologie, Geburtshilfe & Hebammen. We have over one million books available in our catalogue for you to explore.
Information
1
Preinvasive Disease of the Cervix
L. Stewart Massad MD
Key Points
1. Human papillomavirus (HPV) persistent expression is required for progression to cancer.
2. HPV vaccination has the potential to eradicate cervical cancer.
3. Cervical cancer screening now relies heavily on HPV testing.
4. mRNA expression is as sensitive but more specific than DNA testing.
5. Screening guidelines have changed dramatically with the use of contesting and increased intervals between screenings.
Cervical cancer was once the most common cancer in women. It is among the most preventable cancers, and it has become rare among women who engage in cervical cancer prevention programs. Nevertheless, with some 100,000 preinvasive lesions diagnosed in the United States annually, it remains a substantial threat. After tremendous gains following introduction of cytology screening half a century ago, cervical cancer rates continue to fall by about 1% annually. Careful compliance with evidence-based guidelines remains critical to sustaining progress. Effective programs reflect organized public health efforts encompassing patient and clinician education, vaccination against causative types of human papillomavirus (HPV), cytology and HPV screening, colposcopy triage for abnormal screening test results, and destruction of the at-risk cervical transformation zone for women with cancer precursors.
Natural History
Essentially all cervical cancers arise from persistent genital HPV infections (Fig. 1.1). The International Agency for Research on Cancer has designated as carcinogenic 12 HPV types: HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, and -59. As described by Halec and associates, another eight types have been designated as possibly or probably carcinogenic: HPV–26, -53, -66, -67, -68, -70, -73, and -82. Almost 200 HPV types have been identified. A new genotype is based on DNA sequencing. A new type must share less than 90% DNA homology in the L1, E6, and E7 compared with known HPV types.
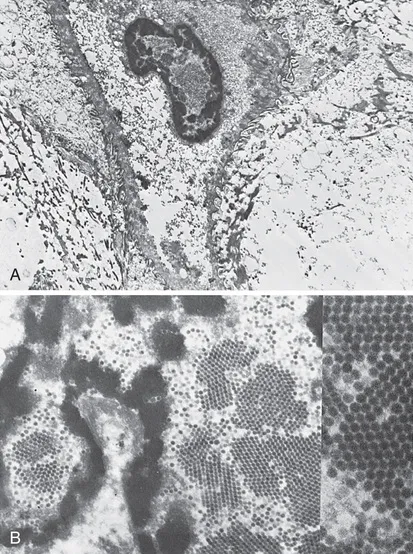
FIGURE 1.1 A, Koilocytotic cells with intranuclear virions (×6900). B, Human papillomavirus particles. Note the intranuclear crystalline array (“honeycomb”) arrangement of virions (×20,500). See the insert (×80,000). (Courtesy of Alex Ferenczy, MD, Montreal, Canada.)
HPV-16 is the most oncogenic, accounting for more than 50% of cervical cancers. HPV-18 is found in 10% of cervical cancers and plays a particularly important role in adenocarcinogenesis. Types 31, 33, and 45 each account for around 5% of cancers. The other types are less oncogenic but have been reported in large typing studies of cervical cancers. HPV-18 and related HPV-45 are linked to cancers found at a younger age.
HPV infection leads to cancer through multiple pathways, but interaction of the HPV E6 and E7 gene products with p53 and pRb are critical: By inactivating or activating degradation of their targets, E6 and E7 eliminate genetic surveillance and allow unchecked cell cycling, leading to accumulation of mutations and eventual invasive cancer. HPV-16 E6 and E7 bind their targets with greater affinity than other HPV types; this may partly explain its greater oncogenicity. Persistent infections lead to cancer in steps: Initial infection into basal epithelial cells leads to establishment of a ring chromosome from which carcinogenic proteins are elaborated while virion production occurs in maturing epithelium. Disruption of the ring, often at the HPV E2 regulatory region, allows integration of E6 and E7 sequence into the host genome. The accumulation of mutations leads to nuclear changes visible cytologically as a high-grade squamous intraepithelial lesion (HSIL) and histologically as high-grade cervical intraepithelial neoplasia (CIN) (Fig. 1.2) is apparent histologically. Selection for invasiveness and metastasis through additional mutation and through gene methylation results in evolution to cancer. Multitype infections do not appear to increase cancer risk, and when multitype infections include HPV-16, most lesions are caused by HPV-16. Extant HPV infections do not appear to predispose to or protect from infection by unrelated types.
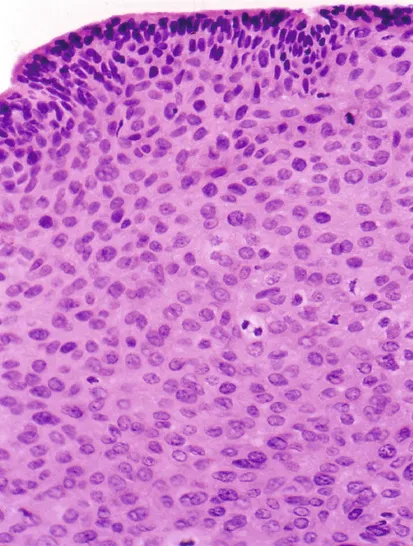
FIGURE 1.2 A cervical intraepithelial neoplasia lesion with multiple mitotic figures.
Vertical transmission of HPV from mother to infant has been documented in the Finnish HPV Family Study but does not appear to result in cervical infection, with genital HPV in only 1.5% of infants after 2 years; fathers' HPV infections did not increase infant HPV risk. Although lifetime abstinence protects against genital HPV infection, nonpenetrative sexual behaviors may transmit the virus, and male exposures modulate female risk. For example, spouses of men who engaged in sex with prostitutes were at higher risk for cervical cancer than those of men who did not, and cervical cancer risk is higher among women whose husbands had more sexual partners. Women who report recent sex only with women are also at risk, though their risk may be marginally lower than that of heterosexual women. Condom use is not fully protective against HPV infection because condoms fail to cover wide areas of genital skin, though it speeds clearance of HPV infections. Male circumcision also reduces but does not eliminate HPV and cancer risks. For these reasons, all women with prior sexual experience, including those who have not been sexually active for years, remain at risk for cervical cancer and merit screening until they have multiple negative test results.
Despite the high frequency of HPV infection, most women infected with carcinogenic HPV, including those with HPV-16, do not develop cervical cancer. Instead, most infections are cleared immunologically. HPV is an intraepithelial virus, and clearance appears to require recognition of infection by cell-mediated immune cells. Roughly half of new infections are cleared within 6 months, with half of the remainder cleared by the end of the first year after infection. Clearance is associated with greater density of CD8+ cells and lower density of T-regulatory cells in underlying stroma. Cervical treatment speeds clearance and reduces risk for posttreatment acquisition of new HPV infections. The type distribution of HPV infection after hysterectomy shows that HPV-16 and HPV-18 have a greater predilection for cervical rather than vaginal epithelium, with HPV types of lesser oncogenicity dominating in the posthysterectomy vagina.
HPV persistence is required for progression of infection to cancer, and women who clear their infections are at low risk. New infections in older women typically do not progress to preinvasive disease or cancer, and women who clear carcinogenic HPV infections have low risk for reappearance with subsequent high-grade CIN. These findings have important implications for termination of screening. Nevertheless, aging appears to result in immune senescence, with many HPV infections in older women attributable to reactivation of previously acquired by latent infections. Oral contraceptive use reduces clearance.
Although determinants of HPV persistence and progression of HPV infection to invasive cancer are poorly understood, several risk factors are known. HPV infection of a cervix undergoing active metaplasia increases risk, as reflected by the epidemiologic observations that early onset of first intercourse is associated with cancer. Smoking is linked to both CIN and cervical cancer. Benzopyrenes have been identified in cervical mucus, and the interaction of tobacco carcinogens with carcinogenic HPV increases risk substantially. Smoking also reduces immune-mediated HPV clearance. Cervical adenocarcinoma and adenocarcinoma in situ (AIS) have been linked to oral contraceptive use. Deficiencies in nutrients such as folate have been linked to cervical oncogenesis but are uncommon among US women. Variants of common HPV types that segregate by ethnicity and polymorphisms in genes related to HPV immune recognition or HPV protein products also modulate HPV persistence and carcinogenic progression. Perhaps most important, lack of screening is a high risk factor for progression of HPV infection to precancer and cancer: Whereas appropriately screened women with multiple risk factors are at relatively low risk, women with few risk factors who are not screened are at higher risk.
Immune factors play a clear role in the clearance or persistence of HPV-related cervical lesions, but the nature of immune defects is poorly understood. Fukuda and associates showed that lesions that persist have fewer Langerhans cells and helper T cells than lesions that are cleared, and tobacco smoking also lowers Langerhans and helper T-cell numbers. In contrast, Molling and associates showed that, although natural killer cells are decreased, regulatory T-cell numbers are increased in women with persistent HPV-16. Immunosuppression related to coinfection with the human immunodeficiency virus (HIV-1) illustrates the importance of immunity in the typical control of HPV. Women with HIV have much higher rates of HPV infection, including multitype infections. HPV clearance rates are lower, although most women do clear their HPV infections if observed long enough, especially if immune reserve as measured by CD4 lymphocyte count remains above 200/cmm. Although most HPV infections in HIV-seropositive women are cleared to such low levels of viral expression that they become nondetectable even with sensitive assays, reactivation appears to occur. This is apparent in cohort studies as the reappearance of previously cleared infections in women who deny sexual activity, often because of illness. Risks in other immunosuppressed states appear to be similar.
HPV infection predicts risk for subsequent high-grade CIN, even among cytologically normal women. In most cases, persistent HPV infections result first in cytologically detectable abnormalities and then in colposcopically visible lesions that grow laterally before developing into invasive cancers. The 10-year risk of high-grade CIN after a single detected HPV infection exceeds 10%.
As developed by Richart through observational studies of the cervix using cytology and colpomicroscopy, a diagnosis of CIN was...