
- 320 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Essential Chemistry for Aromatherapy
About this book
This new edition of ESSENTIAL CHEMISTRY FOR SAFE AROMATHERAPY provides an accessible account of the key theoretical aspects of chemistry and their application into the safe practice of aromatherapy. For readers with a limited science background, this book offers a clear and concisely written guide to essential information in chemistry. For practitioners, the book applies chemistry to the practical and therapeutic use of essential oils, and leads to a better understanding of composition, properties and technical data related to essential oils.- Takes the fear and mystery out of chemistry for aromatherapy students!- Presents crucial information in a clear and easily-digestible format, highlighting key points all along- Allows professional aromatherapists to practice with greater confidence, safety and skill, and to extend the range of their practice through a clearer understanding of chemical properties of essential oils.- Covers the scope of what is taught at major aromatherapy teaching centres, and structures the material to make sure each chapter provides the reader with a rounded understanding of the topic covered.- A glossary is included for easy reference.•Fully-updated and throughout•Chapter 5, Analytical Techniques completely brought up to date•Chapter 6 Oil Profiles updated to include those used in current training•New section entitled 'In perspectives' covers risks and benefits, interpretation of clinical trials and experimental data, use of essential oils in aromatherapy and functional groups in relation to therapeutic properties
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
AROMAFACT
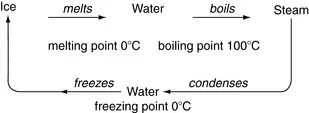
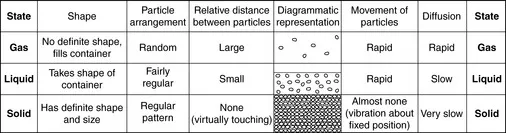
ORGANIZATION OF MATTER
Phases
AROMAFACT
AROMAFACT
AROMAFACT
Physical changes
Chemical changes
Table of contents
- Cover
- Title Page
- Copyright
- Preface
- Acknowledgements
- Table of Contents
- Introduction
- Chapter 1: Fundamentals of chemistry
- Chapter 2: Organic chemistry
- Chapter 3: Families of compounds that occur in essential oils
- Chapter 4: Processing, extraction and purity
- Chapter 5: Analytical techniques
- Chapter 6: Health, disease and therapy
- Chapter 7: Composition of essential oils and other materials
- Chapter 8: Handling, safety and practical applications for use of essential oils
- Bibliography and sources of information
- Glossary
- Index