
- 256 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Botulinum Toxin in Facial Rejuvenation E-Book
About this book
Now thoroughly revised to reflect state-of-the-art advances in the field, Botulinum Toxin in Facial Rejuvenation, 2nd Edition, covers the entire range of the use of botulinum toxin for cosmetic purposes. Dr. Kate Coleman offers practical guidance for safe handling, selection and assessment of patients, potential complications and pitfalls, and aesthetic techniques, as well as comparative modalities and long-term management. This is an ideal resource for anyone who offers this sought-after procedure, including cosmetic surgeons, oculoplastic surgeons, dermatologists, physician's assistants, and registered nurses.
- Features new, unique coverage of long-term management, picturing the same original patients 15 years later, as well as observations on how treatments should be adjusted as the patient gets older in order to respond to natural changes in bone density and underlying support structures.
- Presents new knowledge on neuromodulation and how treatment can be used to 'retrain' expressions to provide fewer frowns lines and better facial symmetry.
- Offers comparative information on other modalities such as laser and hyaluronic acid, as well as potential risk factors, so you can choose the best procedure for each patient.
- Discusses the various forms of botulinum toxin currently available on the market, with an emphasis on Botox, Xeomin, and Dysport.
- Uses full-color clinical photos of pre-, peri-, and post-operative results to illustrate nuances of techniques as well as the effectiveness of botulinum toxin on wrinkles and scars for the major facial areas.
- Provides current guidelines on treatment methods and best practices for reconstitution and storage.
- Discusses which patients may be at risk for adverse effects ?or "worsening results"?and offers suitable alternatives.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Botulinum Toxin in Facial Rejuvenation E-Book by Kate Coleman in PDF and/or ePUB format, as well as other popular books in Medicine & Plastic & Cosmetic Medicine. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Historical background
Abstract
Botulinum toxin comes from Clostridium botulinum bacteria. Type B was discovered in 1910 and type A in the 1920s. It was researched for chemical warfare, and in 1982 Dr. Alan Scott published results on its use around the eye. Botox is produced by Allergan from the original strains explored by Dr. Scott. Dysport evolved from the Porton Down laboratories, United Kingdom, when bought by Ipsen Pharmaceuticals. The author began using Dysport and Botox in the late 1980s to eliminate hyperactive wrinkles on the faces of patients with facial paralysis and hemifacial spasm.
Keywords
Botox; Dysport; Wrinkles; Botulinum toxin
The Clostridium family of bacteria, common to most environments, produces spores that, on germination, release some of the deadliest toxins known to mankind. Clostridium Perfringens contaminates wounds and caused the infamous gas gangrene of World War 1; Clostridium botulinum produces botulinum toxin (BTX)—a powerful neurotoxin.
Clostridium botulinum was first identified in 1897, in Belgium, by Professor Emile van Ermengenen, who was investigating fatal cases of food poisoning following the consumption of macerated ham. It was named after the disease it causes, botulism, a lethal form of food poisoning originally associated with sausage meat (botulus is Latin for sausage). In the same year, an antiserum for botulism was made.
There are seven known serotypes of BTX (A, B, C, D, E, F and G). Serotypes A, B and E cause the classic foodborne disease with a flaccid paralysis of motor and autonomic nerves. Type B was first discovered in 1910, and the isolation of type A began in the 1920s. During the Second World War, research continued into this potent neurotoxin as a possible agent (‘agent X’) for biological warfare. Most of this work was carried out at the chemical warfare laboratories of Fort Detrick, Maryland, and Porton Down in the United Kingdom. Porton Chemicals was bought by Ipsen Pharmaceuticals in 1989 and is the source of Dysport.
Dr Alan Scott, an ophthalmologist from the Smith-Kettlewell Eye Research Foundation, became interested in substances that caused transient muscular paralysis. He acquired BTX type A from Fort Detrick and performed the first clinical tests on humans in 1978. His results in the treatment of strabismus (an abnormal contraction of the extraocular eye muscles) were published in 1982 and led to the extensive use of BTX type A by ophthalmologists in the treatment of blepharospasm (an abnormal twitching and contraction of the muscles around the eye), hemifacial spasm and cervical dystonia.
By the late 1980s, the author, an oculofacial and ophthalmic surgeon in Ireland, as well as colleagues in Canada and the United Kingdom, were each exploring different doses and methods for using BTX. The author worked on patients with facial asymmetry, especially Bell palsy, to calculate the doses required to ‘balance’ the innervation of the whole face.
Thereafter the story is well known. With good medical training and the correct selection of patients, BTX can be given easily, safely and repeatedly. There have been relatively few reported side effects despite ever-increasing demand in increasingly large doses, in particular for large muscle dystonia and spasm.
Thanks to Dr Scott, many lives have been enhanced and many more made bearable by this deadliest of poisons and astonishingly precise drug.
Chapter 2
Botulinum toxin: Mode of action and serotypes
Dr. Kate Coleman, BSc PhD FRCS FRCOphth
Abstract
Botulinum toxin comprises a light and a heavy chain protein molecule. The heavy chain attaches the molecule to the nerve membrane, allowing the light chain to reach the site of action at the protein complex in the nerve ending. The toxin inhibits the release of acetylcholine at the neuromuscular junction and can inhibit contraction for 12 weeks before new nerve endings bud and restore function. The autonomic cholinergic receptors are also blocked, for up to 12 months. Central action can result from anterograde and retrograde axonal transport with secondary reduction of elements of the basal ganglia. Antinociceptive effects are due to blockades of pain and inflammatory mediator release. The different serotypes act on different elements of the vesicle-associated membrane protein/synaptosomal-associated protein-25/syntaxin protein complex. The development of antibodies to the protein complex is reported in 2% with serotype A (BOTOX) as opposed to 20% to 40% with serotype B (Myobloc).
Keywords
BOTOX; Dysport; neurotoxin; antinociceptive; serotype
A working knowledge of the pharmacology of botulinum toxin (BTX) is essential to understand the contraindications and complications of treatment with it.
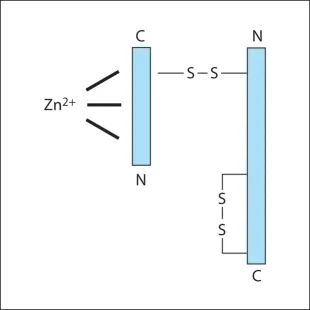
Botulinum neurotoxins are metalloprotease polypeptides, comprising a protein molecule (150 Kd), which can be cleaved enzymatically into a heavy (H) (100 Kd) and a light (L) (50 Kd) chain (Fig. 2.1). These chains are normally held together by a disulphide bond, which is heat labile. Disruption of this bond inactivates the neurotoxin. This explains why BTX must be stored at the correct temperature and reconstituted carefully, preserving the integrity of the two-chained molecule. Prior to reconstitution, characteristics of Incobotulinum toxin A (Xeomin) reflect the lack of a complexing protein with the neurotoxin, allowing lpng term stability and reduced immunogenicity.
BTX induces paralysis by blocking the release of acetylcholine at the skeletal alpha motor neurone neuromuscular junction, thereby inhibiting the transmission of nerve impulses across the synaptic junction to the motor end plate.
Muscular contraction: Normal cholinergic transmission (fig. 2.2)
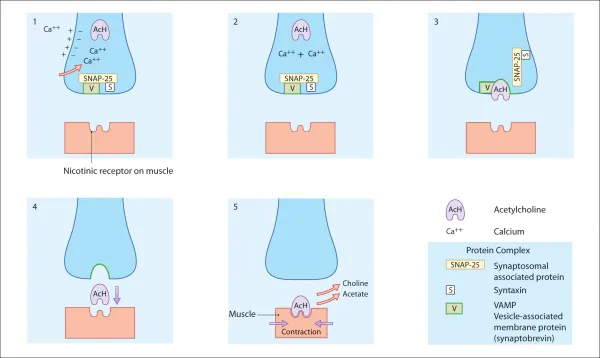
Voluntary muscle contraction is a response to stimulation by action potentials passing along a nerve to the muscle. Once these action potentials reach a synapse at the neuromuscular junction, they stimulate an influx of calcium into the cytoplasm of the nerve ending. This increase in calcium concentration allows acetylcholine to fuse with the membrane, using a protein complex, before crossing the synapse and fusing with nicotinic receptors on the muscle fibre. The protein complex consists of three types of protein: vesicle-associated membrane protein (VAMP; synaptobrevin), synaptosomal-associated protein (SNAP)-25 and syntaxin.
Mode of action of botulinum toxin (fig. 2.3)
Acetylcholine depends on a protein complex for its release from the nerve ending into the synapse. BTX, using a specific enzyme in the L-chain, interacts with one component of the protein complex of the nerve terminal, thereby inhibiting the discharge of the acetylcholine. The protein attacked is specific to the different serotypes of BTX; for example BTX-A blocks SNAP-25, whereas BTX-B blocks VAMP. BTX-B acts on a different cytoplasmic protein complex. The secretion of acetylcholine is disrupted when the L-chain of the BTX-B molecule cleaves a protein called synaptobrevin, also known as VAMP. Clinical trials have shown BTX-B to be effective for the treatment of patients with cervical dystonia, including those resistant to BTX-A.
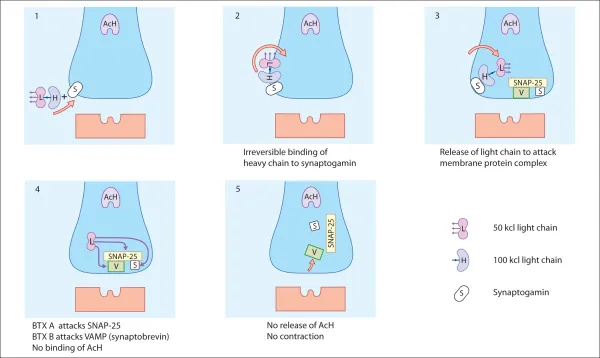
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Foreword to the First Edition 2003
- Appreciation
- Introduction
- 1. Historical background
- 2. Botulinum toxin: Mode of action and serotypes
- 3. Clinical indications and use
- 4. Patient preparation and injection skills
- 5. Patient selection: The art of understanding neuromodulation and symmetry
- 6. The art of patient selection and short- and long-term management
- 7. Contraindications and complications
- 8. Management of crow’s feet
- 9. Management of forehead wrinkles
- 10. Treatment of the perioral region, the neck and scars
- 11. Other solutions
- Index