People interact (i.e., engage) with the world around them using their senses. Accordingly, when we interact with medical devices, such as a dialysis machine, syringe, infusion pump, or surgical stapler, we also use our senses. The parts of any such device that we see, hear, touch, smell, or taste comprise the user interface. Accordingly, the user interface might include hardware components, software screens, labeling (including user documentation), soundscapes, aromas (odors), material that literally has a taste, and more.
As illustrated in the following section, user interfaces may be one particular type or many (i.e., a hybrid).
HARDWARE
Here are some examples of hardware user interfaces or components therein, noting that the overall product might be a hybrid of hardware, software, and labeling.
| Examples | |
| Rotary knobs used to adjust the gas mixture (i.e., the amount of air, nitrous oxide, and oxygen) | |
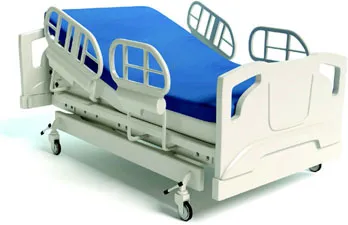
| Guardrail (bed rail) that keeps a patient from falling out of a hospital bed | |
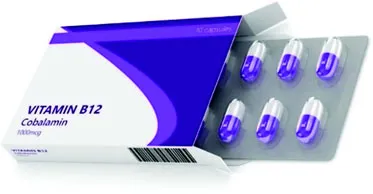
| Package and blister pack containing vitamin capsules | |
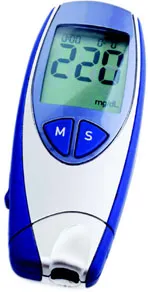
| Pushbutton (“M,” for memory) used to recall and display the most recent measurement recorded by a glucose meter | |
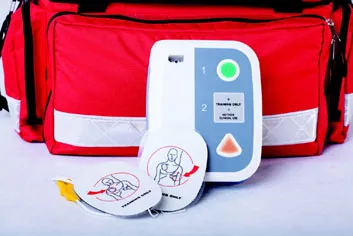
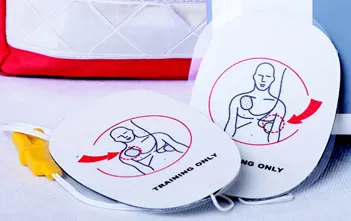
| Training-only electrode pads used to simulate delivering a cardioverting shock using an automated external defibrillator | |
| |
SOFTWARE
Even though a computer display is technically a hardware component, the display and the information presented on it (i.e., on screens) are normally referred to as the software user interface. Generally speaking, people use their eyes and a pointing device (e.g., mouse, fingertip, trackpad, stylus) to interact with information presented on a screen. Notably, a device’s software user interface may incorporate more than one display.
| Examples | |
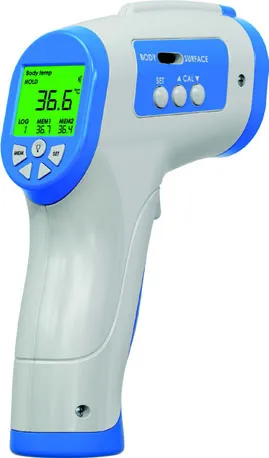
| Small, segmented LCD display that presents the temperature measurement taken by a forehead digital thermometer | |
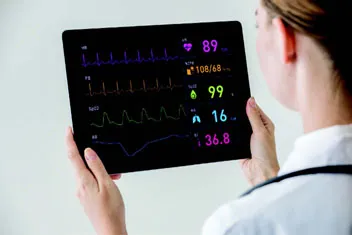
| Tablet LCD display that presents hemodynamic parameter values and waveforms on a patient monitor | |
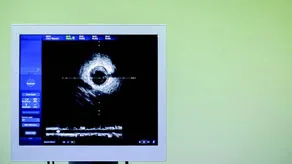
| Large LED display that presents images of the coronary arteries, captured by an intravascular ultrasound imaging system | |
| Touchscreen display, which displays information and touch controls, is built into a blood hematology analyzer | |
| |
| Smartphone and smartwatch screens display a patient’s sinus rhythm data, which is analyzed to determine if there is atrial fibrillation | |
| |
LABELING
Labeling is a term of art in the medical industry, referring to the words and symbols that might appear on hardware and software user interfaces as well as the various types of documents (virtual and printed) that accompany a medical device. Here are some examples.
| Examples | |
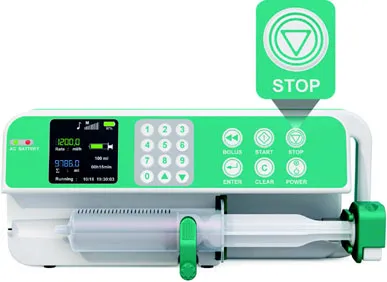
| Stop symbol printed on a syringe infusion pump’s button | |
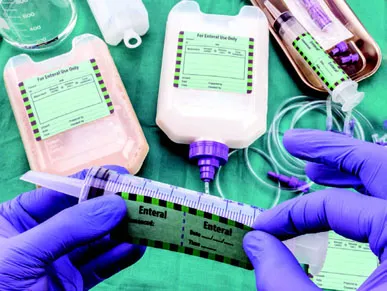
| Enteral feeding product labels that enable clinicians to document the time and date | |
| “Getting started guide” that introduces new users to a colon cancer screening kit | |
| User manual that thoroughly describes a tonometer’s operation | |
| Package insert that provides step-by-step guidance for performing a COVID-19 test | |
SOUNDSCAPES
The sound generated by a device, which might be produced by a speaker or contact between physical components, is also a user interface element. We refer to such stimuli as a soundscape. A soundscape may include the following types of s...