
Basic Laboratory Methods for Biotechnology
Textbook and Laboratory Reference
- 1,172 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Basic Laboratory Methods for Biotechnology
Textbook and Laboratory Reference
About this book
Basic Laboratory Methods for Biotechnology, Third Edition is a versatile textbook that provides students with a solid foundation to pursue employment in the biotech industry and can later serve as a practical reference to ensure success at each stage in their career. The authors focus on basic principles and methods while skillfully including recent innovations and industry trends throughout.
Fundamental laboratory skills are emphasized, and boxed content provides step by step laboratory method instructions for ease of reference at any point in the students' progress. Worked through examples and practice problems and solutions assist student comprehension. Coverage includes safety practices and instructions on using common laboratory instruments.
Key Features:
-
Provides a valuable reference for laboratory professionals at all stages of their careers.
- Focuses on basic principles and methods to provide students with the knowledge needed to begin a career in the Biotechnology industry.
- Describes fundamental laboratory skills.
- Includes laboratory scenario-based questions that require students to write or discuss their answers to ensure they have mastered the chapter content.
- Updates reflect recent innovations and regulatory requirements to ensure students stay up to date.
- Tables, a detailed glossary, practice problems and solutions, case studies and anecdotes provide students with the tools needed to master the content.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Unit V Obtaining Reproducible Laboratory Measurements
- Chapter 15: Introduction to Quality Laboratory Measurements
- Chapter 16: Introduction to Instrumental Methods and Electricity
- Chapter 17: The Measurement of Weight
- Chapter 18: The Measurement of Volume
- Chapter 19: The Measurement of Temperature
- Chapter 20: The Measurement of pH, Selected Ions, and Conductivity
- Chapter 21: Measurements Involving Light – Part A: Basic Principles and Instrumentation
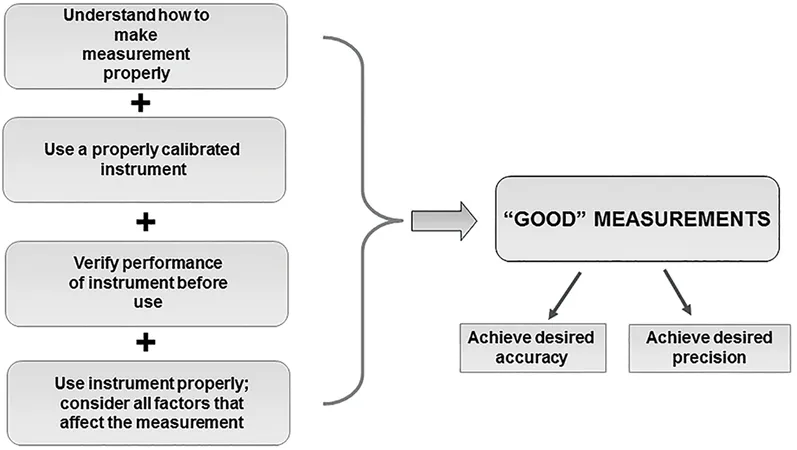
- Chapter 15 introduces basic principles underlying measurements and terminology relating to metrology (the study of measurements).
- Chapter 16 introduces basic principles of electricity and electronics, and instrumental methods of measurement. This chapter serves as a transition to the next five chapters, each of which discusses a specific type of measurement.
- Chapter 17 introduces principles and practices relating to weight measurements in the biotechnology laboratory.
- Chapter 18 introduces principles and practices relating to volume measurements in the biotechnology laboratory.
- Chapter 19 introduces principles and practices relating to temperature measurements in the biotechnology laboratory.
- Chapter 20 introduces principles and practices relating to the measurement of pH, selected ions, and conductivity.
- Chapter 21 introduces principles and practices relating to the measurement of light transmittance and absorbance.
Bibliography for Unit V
Table of contents
- Cover
- Half Title Page
- Title Page
- Copyright Page
- Table of Contents
- Preface
- Ancillary Materials
- Acknowledgements
- Authors
- Unit I Biotechnology Is the Transformation of Knowledge into Useful Products
- Unit II Introduction to Quality in Biotechnology Workplaces
- Unit III Safety in the Laboratory
- Unit IV Math in the Biotechnology Laboratory: An Overview
- Unit V Obtaining Reproducible Laboratory Measurements
- Unit VI Laboratory Solutions
- Unit VII Quality Assays and Tests
- Unit VIII Cell Culture and Reproducibility
- Unit IX Basic Separation Methods
- Unit X Biotechnology and Regulatory Affairs
- Appendix: Answers to Practice Problems
- Acronyms
- Glossary Terms
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app