
The Chemistry and Mechanism of Art Materials
Unsuspected Properties and Outcomes
- 172 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
This unique book presents an integrated approach to the chemistry of art materials, exploring the many chemical processes involved. The Chemistry and Mechanism of Art Materials: Unsuspected Properties and Outcomes engages readers with historical vignettes detailing examples of unexpected outcomes due to materials used by known artists.
The book discusses artists' materials focusing on relevant chemical mechanisms which underlie the synthesis and deterioration of inorganic pigments in paintings, the ageing of the binder in oil paintings, and sulfation of wall paintings as well as the toxicology of these pigments and solvents used by artists. Mechanisms illustrate the stepwise structural transformation of a variety of art materials.
Based on the author's years of experience teaching college chemistry, the approach is descriptive and non-mathematical throughout. An introductory section includes a review of basic concepts and provides concise descriptions of analytical methods used in contemporary art conservation.
Additional features include:
- Illustrations of chemical reactivity associated with art materials
- Includes a review of chemical bonding principles, redox and mechanism writing
- Covers analytical techniques used by art conservation scientists
- Accessible for readers with a limited science background
- Provides numerous references for readers seeking additional information
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1 Essential Concepts
1.1 Chemical Bonding
| Solid/property | Metallic | Ionic | Molecular | Network |
|---|---|---|---|---|
| Particle | Atoms | Ions | Molecules | Atoms |
| Bonding | Metallic | Ionic | Covalent | Covalent |
| Melting point | Low to high | High | Low | High |
| Elec. Conductivity | High | Solid: nil | Nil | Nil |
| Melt: high | ||||
| Solution: high | ||||
| Hardness | Hard | Hard | Soft | Hard |
| Solubility | Insol | Depends on ionic charge | Like dissolves like | Insoluble |
| Examples | Ag, Cu, Pb | CaCO3 | Arsenic sulfides | Diamond |
| Prussian blue | Orpiment, realgar | Silica |
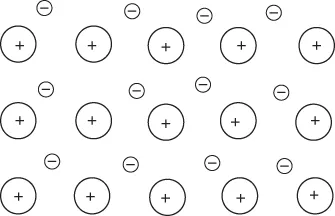
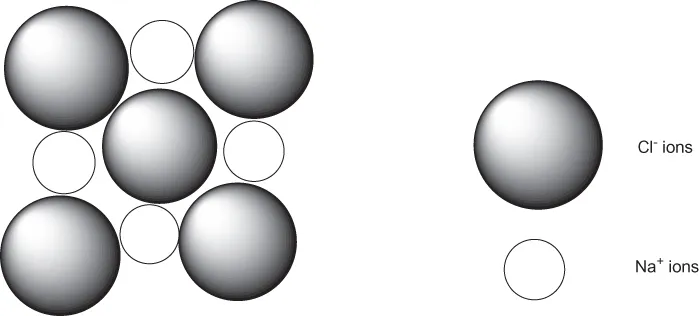
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- List of Figures
- List of Schemes
- List of Tables
- Preface
- Acknowledgments
- Author
- List of Abbreviations
- Chapter 1 Essential Concepts
- Chapter 2 Preparation of Inorganic Pigments Used by Artists
- Chapter 3 Silica, Silicates and Aluminosilicates
- Chapter 4 Discoloration Stories
- Chapter 5 Toxicology of Art Materials
- Chapter 6 Aging of Oil Paint Binder
- Chapter 7 Aging of Wall Paintings
- Index