1.1 Introduction
The central nervous system (CNS) consists of the spinal cord and the brain is the sophisticated body system that controls most of the body's functions. The complex structure, function, and operating efficiency of the mysterious brain have attracted philosophers, scientists, and engineers to gain deeper insights and emulate them for biomedical and technological advancements. Advanced neurological and behavioral research has revealed some of the structural and functional mysteries of the brain and associated disease mechanisms. The brain cells (neurons) are highly specialized in establishing complex connections and networks to efficiently relay the sensory signals and information to-and-fro from the brain to coordinate the physiological or psychological body functions. The loss of neuronal functions and developing atrophy due to neurodegeneration is the leading cause of several neurodegenerative disorders and the condition is collectively termed dementia. Dementia is characterized by the symptoms of impairment in memory, communication, behavior, and thinking that severely affect the ability to perform day-to-day activities. More than a century ago, Alois Alzheimer, a German physician, described a unique progressive neurodegeneration, which was subsequently named Alzheimer's disease (AD) by his colleague Emil Kraepelin.1,2
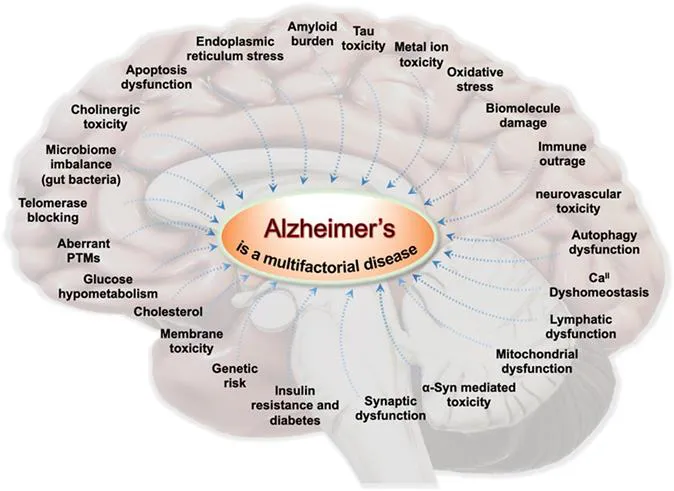
AD is a chronic neurodegeneration-related disorder and rapidly growing health calamity worldwide with 70–80% contribution to all dementia cases.3 Aging is one of the leading risk factors of AD, while genetic factors and family heredity are known to have a definite role. The absence of accurate diagnosis and treatment is contributing to its fast rise as a global health and economic burden. There are currently ∼50 million people suffering from AD, and this number is expected to cross over 130 million by 2050.4 Besides, the statistics reveal that the number of deaths by AD increased by ∼146% between 2000 and 2018, while all other major diseases such as heart diseases, HIV, and cancer showed an appreciable decrease owing to the availability of improved detection and treatment options.2 The evidence accumulated through multidisciplinary academic and clinical studies have indicated the multifactorial nature of AD (Figure 1.1). The multifactorial nature characterized by multiple toxicities hampers the development of accurate diagnosis and disease-modifying therapies.5 There are two types of AD, namely (i) early-onset, a rare form of AD contributing to <5% of total AD, and (ii) late-onset, the most prevalent form of AD. Both AD types show a multifactorial nature and are associated with multifaceted toxicity.3,4 The abnormalities linked to various vital physiological processes are responsible for the multifaceted toxicity, which includes cholinergic toxicity, amyloid burden, tau toxicity, metal ion toxicity, oxidative stress, biomolecular damage, immune outrage, calcium ion (CaII) dyshomeostasis, neurovascular toxicity, lymphatic dysfunction, mitochondrial dysfunctions, α-synuclein mediated toxicity, synaptic dysfunctions, membrane toxicity, apoptosis dysfunctions, impairment in telomerase activity, aberrant post-translational modifications, microbial imbalance and infection, glucose hypometabolism, endoplasmic reticulum stress, the role of cholesterol, autophagy dysfunctions, genetic risk, and insulin resistance and diabetes among others. This chapter provides a concise overview of AD onset and progression concerning various individual pathophysiological processes and their interplay (Figure 1.1). We briefly refer to the current status of biomarkers, diagnosis, targets, and therapy as a prelude to the detailed discussion of each topic in the subsequent chapters.
Figure 1.1 Various pathophysiological events in the brain that concurrently play crucial roles in the multifactorial AD pathogenesis and progression.
1.2 Pathological Events
1.2.1 Amyloid Burden
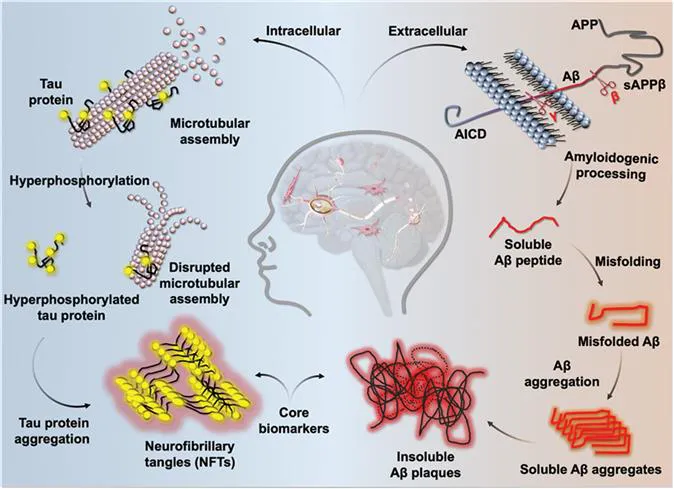
The amyloid burden plays a central role in AD pathogenesis.6 Decades of academic and clinical research have established that the accumulation of amyloidogenic amyloid beta (Aβ) and its deposition in the brain is a major event in the etiology of AD.6,7 The pathogenic mutation in the amyloid precursor protein gene (chromosome 21) encoded for essential transmembrane neuronal glycoprotein, amyloid precursor protein (APP) was identified. The proteolysis of APP by the specific combination of proteases (secretases) leads to generation, misfolding, self-aggregation, and deposition of insoluble Aβ plaques in the AD brain.7 Normally, the expression of unmutated APP is associated with neurite development, cell growth, ions transport, and healthy synapse.8 The proteolysis of APP through α-secretase and γ-secretase generates soluble and nontoxic peptide fragments that are cleared from the healthy brain. Under the pathologically prone conditions, mutated APP undergoes sequential proteolysis by β-secretase and γ-secretase to produce Aβ peptides of variable length (37–43 amino acids).6–8 Aβ peptides (mainly Aβ42) are highly aggregation-prone and undergo self-assembly to form toxic polymorphic aggregation species (alloforms) viz., oligomers, protofibrils, and insoluble fibrils. As a result, the concentration levels of Aβ monomers rise and decline in the brain and cerebrospinal fluid (CSF), respectively, with the disease progression.3 Accumulating evidence has demonstrated that oligomers are the most toxic form of Aβ compared to protofibrils or fully grown fibrils (Figure 1.2).4 In other words, the surface of fully grown fibrillar aggregates acts as an active catalytic surface to produce highly toxic Aβ oligomers through the secondary nucleation process.9 The toxic Aβ aggregation species interact and damage the neuronal plasma membrane, which prompts the internalization of misfolded Aβ peptides into the healthy neurons.3 The insoluble Aβ aggregation species are highly neurotoxic to mature neurons as they cause axonal and dendritic atrophy.7 Aβ oligomers disrupt neuronal signaling cascade leading to synaptic dysfunction, weakening in neuronal circuits, and neuronal death. The N-methyl-d-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionicacid (AMPA) receptors are blocked by Aβ aggregation species causing memory impairment and cognitive dysfunctions. In addition, regulation of endocytosis and surface expression of NMDA-type glutamate receptors by Aβ reduce the synaptic plasticity and hippocampal long-term potentiation (LTP) formation.10 As a result, Aβ is designated as one of the definite biomarkers by the National Institute on Aging and Alzheimer's Association (NIA-AA) Research Framework 2018, and the levels of Aβ species are directly correlated with the disease progression and decline of cognitive functions in AD.11 Further, the amyloid burden is closely associated with various other pathological events such as metal dyshomeostasis, excess ROS production, oxidative stress, inflammation, mitochondrial damage, tau phosphorylation, and aggregation among other neurotoxic processes (Figure 1.2).3 Overall, the involvement of Aβ burden is widely studied and accepted for understanding the multifactorial nature of AD and became a promising therapeutic target to modify AD pathology (see Chapter 16).12–16
Figure 1.2 The major intracellular and extracellular pathological events (tau and Aβ) under progressive AD conditions. These pathological changes in the AD brain provide core biomarkers for AD diagnosis.
1.2.2 Tau Toxicity
Microtubule-associated proteins (MAPs) are engaged in the maintenance and stabilization of the microtubule assembly in matured neurons. Tau, a MAP, interacts with tubulin and stimulates its assembly into microtubules (Figure 1.2).17 The activity of tau is regulated through the extent of phosphorylation and one mole of tau phosphorylated with 2–3 moles of phosphate under physiological conditions. However, hyperphosphorylation of tau (pTau) reduces its normal physiological function leading to several neuronal disorders like AD and tauopathies (see Chapters 3 and 17).18 The self-aggregation of pTau forms intracellular neurofibrillary tangles (NFTs) and paired helical filaments (PHFs) that impair the axonal functions and degenerate neuronal cells (see Chapters 3–6).18 The pTau significantly reduces the necessary O-glycosylation modifications (of Ser and Thr) and impedes its activity. Initially, the tau hypothesis in AD was considered an independent pathway, while recent studies have established overlapping links with the amyloid hypothesis.4 Aβ-mediated glycogen synthase kinase 3β (GSK3β) activation triggers the tau hyperphosphorylation under AD conditions.19 It has been shown that misfolded tau transport from neuron to neuron and spread in the brain, which is significantly influenced by the amyloid load.20 Further, apolipoprotein E4 (APOE4) increases the accumulation of pTau by decreasing its clearance from the AD brain.21 Considering the prominent role of tau in AD pathology, the NIA-AA Research Framework 2018 has declared tau (pTau and NFT) as one of the core biomarkers (Figure 1.2).11
1.2.3 Metal Ion Toxicity
Metal ions play a crucial role in functioning biological systems and their dyshomeostasis is linked to several disease conditions. In the CNS, bioactive metal ions (CuII, ZnII, and FeIII) serve as cofactors for enzymatic activity, mitochondrial, and neuronal functions.22 However, the accumulation of high concentrations of these metal ions in their free form and as inclusion complexes with insoluble amyloid aggregates aggravate AD pathology. In 1994, Bush et al. showed the possib...