
- 432 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Advanced Electrochemical Materials in Energy Conversion and Storage
About this book
This book focuses on novel electrochemical materials particularly designed for specific energy applications. It presents the relationship between materials properties, state-of-the-art processing, and device performance and sheds light on the research, development, and deployment (RD&D) trend of emerging materials and technologies in this field.
Features:
- Emphasizes electrochemical materials applied in PEM fuel cells and water splitting
- Summarizes anode, cathode, electrolyte, and additive materials developed for lithium-ion batteries and reviews other batteries, including lithium-air, lithium-sulfur, sodium- and potassium-ion batteries, and multivalent-ion batteries
- Discusses advanced carbon materials for supercapacitors
- Highlights catalyst design and development for CO2RR and fundamentals of proton facilitated reduction reactions
With a cross-disciplinary approach, this work will be of interest to scientists and engineers across chemical engineering, mechanical engineering, materials science, chemistry, physics, and other disciplines working to advance electrochemical energy conversion and storage capabilities and applications.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Advanced Electrochemical Materials in Energy Conversion and Storage by Junbo Hou in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemical & Biochemical Engineering. We have over one million books available in our catalogue for you to explore.
Information
1 Catalyst Support Materials for Proton Exchange Membrane Fuel Cells
Department of Mechanical and Energy Engineering, Southern University of Science and Technology, Shenzhen, Guangdong, People’s Republic of China
Department of Materials Science and Engineering, Southern University of Science and Technology, Shenzhen, Guangdong, People’s Republic of China
CONTENTS
- 1.1 Introduction
- 1.2 Functionalized Carbon Support Materials
- 1.2.1 Carbon Nanospheres
- 1.2.2 High Surface Area Carbon
- 1.2.3 Graphitized Carbon Materials
- 1.2.4 Other Carbon Materials (Carbon Nanotubes and Graphene)
- 1.3 Carbide Support Materials
- 1.3.1 Boron Carbide
- 1.3.2 Silicon Carbide
- 1.3.3 Titanium Carbide
- 1.3.4 Tungsten Carbides
- 1.3.5 Molybdenum Carbides
- 1.4 Nitride Support Materials
- 1.5 Transition Metal Oxides
- 1.5.1 Titanium Oxides
- 1.5.2 Tungsten Oxides
- 1.6 Perspectives and Outlook
- References
DOI: 10.1201/9781003133971-1
1.1 Introduction
Proton exchange membrane fuel cell (PEMFC) has been widely employed as electrochemical energy conversion systems in many fields due to its advantages, such as high energy conversion efficiency, high power density, and low operation temperature. Particularly, due to its high power density, PEMFC is suitable for transportation applications, such as sedans and buses. However, its wide commercialization is still hampered by three aspects: high cost, low catalyst activity, and low durability. The high cost is mainly caused by the utilization of platinum catalysts. In the early stage of PEMFC development, platinum black was employed as the catalyst with catalysts loading as high as 28 mg/cm2. In the late 1990s, researchers began to use the high-surface-area carbon to disperse the platinum with a nanosize structure. Hence, the platinum catalyst loading in the catalyst layer was significantly reduced to 0.3–0.4 mg/cm2. In this regard, the catalyst supporting material is critical to improving noble catalyst utilization. Besides the dispersion of nanosized catalysts, the catalyst supporting material is also related to the mass transport and durability of PEMFC. On the one hand, with the catalyst loading further reduced, for example, <0.1 mg/cm2, the mass transport, particularly for the oxygen diffusion in the cathode, arises as a critical issue. Hence, the optimization architecture of catalyst supporting material, including the pore size, pore distribution, and porosity, to meet the mass transport requirement becomes a new research highlight in the research community of PEMFC. On the other hand, the durability of PEMFC highly depends on the catalyst supporting materials. Without a suitable supporting material, the platinum nanoparticles will be agglomerated, dissoluted, and detached, which will deteriorate the performance of PEMFC. Therefore, a suitable catalyst supporting material should meet the following requirements:
- High surface area;
- High electrical conductivity;
- High durability under the harsh chemical environment (high potential and low pH);
- Suitable nanostructure for platinum dispersion and mass transport.
In this chapter, the cutting-edge development of catalyst supporting materials for platinum in the recent decade will be first introduced. These catalyst supporting materials include functionalized carbon, carbide, nitride, and transition metal oxides. The perspective and future development will then be given at the end of the chapter.
1.2 Functionalized Carbon Support Materials
The carbon supports play a critical role in determining catalyst performance and stability [1,2,3]. Porous carbons have internal pores within their primary particles and often have very high surface areas (500–2000 m2/g), suitable for Pt deposition. Due to the internal porosity of porous carbons, many Pt nanoparticles can be deposited in the interior of the micropores [4]. The carbon micropores normally have very narrow openings (1–4 nm), and the pores themselves can be tortuous [5,6]. Electrochemical measurements showed that when the catalyst was fabricated to form the catalyst layer, the ionomer did not penetrate into the small pores to contact these interior Pt nanoparticles [7,8,9,10,11]. On the basis of this physical structure, Figure 1.1 illustrates the kinetic and transport properties of the catalysts using different carbon supports [5]. Solid carbon-supported Pt catalysts have relatively poor activity due to the poisoning of the active sites by the ionomer but have good transport properties. In contrast, on porous carbon catalysts, the interior Pt nanoparticles uncoated by ionomers have good activity but restricted access to protons and reactant gases. The performance loss due to local transport depends on the required local flux of reactants. Therefore, PEMFC with low Pt loading will become more susceptible to this loss at a high power load. Based on the reasons mentioned, excellent carbon support needs to have some important pore characteristics. It should have accessible internal-connected porous structures that can host catalyst nanoparticles and prevent them from direct contact with ionomers but still allow protons and hydrogen/oxygen to have reasonable access to the catalytic species. Furthermore, the pore depth and tortuosity should not be too large. The accessible carbon pores should have a reasonably small opening to restrict ionomer penetration but not too small to restrict oxygen transport to the catalytic sites. The concept is straightforward, but it will not be easy to use a reasonable method to manufacture carbon support that meets all of the requirements.
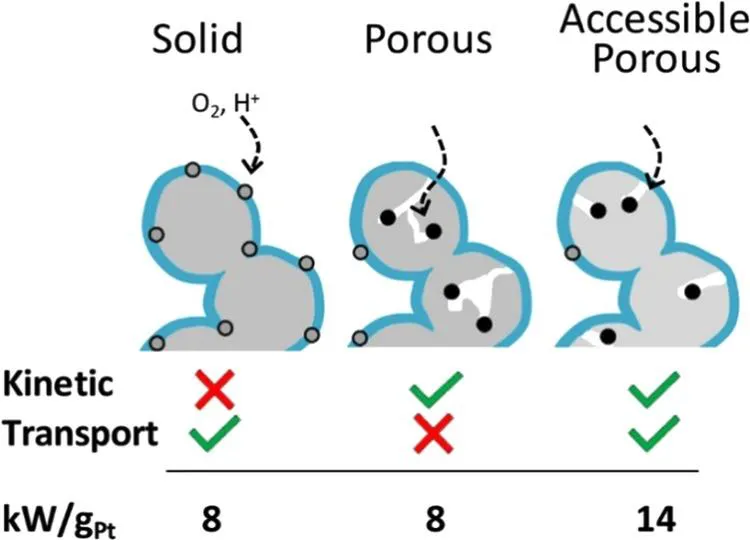
Figure 1.1 ORR kinetic and transport (O2 and proton) characteristics of catalyst layer structures made from three types of carbon (gray). Small black and gray circles represent relatively high and low activity Pt particles, respectively, due to ionomer (blue) adsorption [5].
1.2.1 Carbon Nanospheres
Carbon spheres with a nanometer diameter are widely employed as the catalyst support for PEMFCs. As one of the products manufactured by pyrolyzing petroleum hydrocarbon, carbon nanospheres (or carbon blacks) are the most commonly used supports for Pt-based catalysts for PEMFCs in many studies and commercial applications. Carbon nanospheres consist of near-spherical particles, <50 nm in diameter. These coalesce into particle aggregates and agglomerates of around 250 nm in diameter. Typical carbon nanospheres, such as Vulcan XC-72, acetylene black, and Ketjenblack, possess different physicochemical characteristics (specific surface area, electronic conductivity, the surface-to-volume ratio, stability, and surface functionality). Vulcan XC-72 nanoparticles have attracted special attention due to their excellent compromise between adequate surface area (~250 m2/g) and high electric conductivity (~2.77 S/cm). Acetylene black is a material produced by the incomplete combustion of ethylene cracking tar. Researchers have applied acetylene black as carbon support for a range of applications for many years. Uchida and co-workers reported the high specific activity of Pt/C with acetylene black as carbon support for PEMFCs and direct methanol fuel cells [12,13]. Acetylene black mainly has the following characteristics: (i) low resistivity, (ii) ability to absorb and reserve an effective volume of electrolyte without cutting down its ability to mix with the active material, (iii) high surface area...
Table of contents
- Cover
- Half Title
- Series Page
- Title Page
- Copyright Page
- Contents
- Preface
- Editor
- Contributors
- Chapter 1 Catalyst Support Materials for Proton Exchange Membrane Fuel Cells
- Chapter 2 Recent Advances in Low PGM for Fuel Cell Electrocatalysis
- Chapter 3 Ultralow Pt Loading for a Completely New Design of PEM Fuel Cells
- Chapter 4 Outlines for the Next-Generation Cathode Materials Utilized in Lithium Batteries
- Chapter 5 Cathode Materials for Lithium-Sulfur Batteries
- Chapter 6 Anode Materials for Lithium-Sulfur Batteries
- Chapter 7 Interlayer of Lithium-Sulfur Batteries
- Chapter 8 Principles and Status of Lithium-Sulfur Batteries
- Chapter 9 Solid-State Batteries and Interface Issues
- Chapter 10 Key Electrode Materials for Lithium-Ion Capacitor Batteries
- Chapter 11 Solar-Induced CO2 Electro-Thermochemical Conversion and Emission Reduction Principles
- Chapter 12 CO2 Electrochemical Reduction to CO: From Catalysts, Electrodes to Electrolytic Cells and Effect of Operating Conditions
- Chapter 13 Improving the Electrocatalytic Performance by Defect Engineering and External Field Regulation
- Index