
eBook - ePub
Tuberculosis
The Microbe Host Interface
Larry S. Schlesinger, Lucy E. DesJardin
This is a test
Share book
- 292 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Tuberculosis
The Microbe Host Interface
Larry S. Schlesinger, Lucy E. DesJardin
Book details
Book preview
Table of contents
Citations
About This Book
M. tuberculosis remains one of the most successful human pathogens known. The causative agent of tuberculosis, it also has a unique ability to persist for years in the infected, apparently healthy host. This dormant organism can be reactivated years, even decades later to cause tuberculosis. This book reviews the most important state-of-the-art approaches currently used to study microbe-host interactions and highlights emerging methodologies.
Frequently asked questions
How do I cancel my subscription?
Can/how do I download books?
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
What is the difference between the pricing plans?
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
What is Perlego?
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Do you support text-to-speech?
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Is Tuberculosis an online PDF/ePUB?
Yes, you can access Tuberculosis by Larry S. Schlesinger, Lucy E. DesJardin in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Biology. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
M.tuberculosis Entry and Growth using Macrophage Models
Abstract
Although the central role for mononuclear phagocytes in the pathogenesis of tuberculosis has been known for a century, the resurgence of tuberculosis in the United States and worldwide over the past two decades has lead to an equally impressive resurgence of research aimed at further defining the molecular events underlying many aspects of the M. tuberculosis (MTB)-mononuclear phagocyte interaction. This chapter summarizes recent advances and compares various in vitro and in vivo models, pointing out strengths and weaknesses. It is clear that monocytes and macrophages differ phenotypically and functionally among mammals and between tissue compartments of the human host in ways that impact on the host response to MTB.
Introduction
Tuberculosis (TB) continues to plague mankind, with increasing worldwide mortality and morbidity. The intense interaction of TB with human immunodeficiency virus (HIV) infection over the last two decades has compounded the scene even further, and purports to more global devastation. Understanding the pathogenesis of infection with Mycobacterium tuberculosis (MTB) remains critical to development of new modalities to treat and prevent this disease. As macrophages are the cornerstone of MTB infection, a full understanding of phagocytosis and survival of MTB within this cellular target is needed to achieve this goal.
Humans are the sole natural host of MTB infection. The global infectious reservoir is immense with over 1/3 of the world population infected (Raviglione et al., 1995). Maintenance of the infectious cycle among humans is dependent on the transmission of MTB from cases with active pulmonary TB to immunologically naïve subjects. The extreme susceptibility to MTB infection is reflected by the fact that aerosolization of a few (5-10) bacilli allows for a primary MTB infection (Smith et al., 1965). The initial interaction of MTB with the host involves alveolar macrophages, which remain the only cell type shown to harbor MTB in vivo (Filley and Rook, 1991). Phagocytosis and intracellular growth of MTB within alveolar macrophages initiate the events of primary infection. Activation of components of innate immunity, the recruitment of various classes of lymphocytes and monocytes to sites of infection, and the final development of specific immunity allow for the containment of infection.
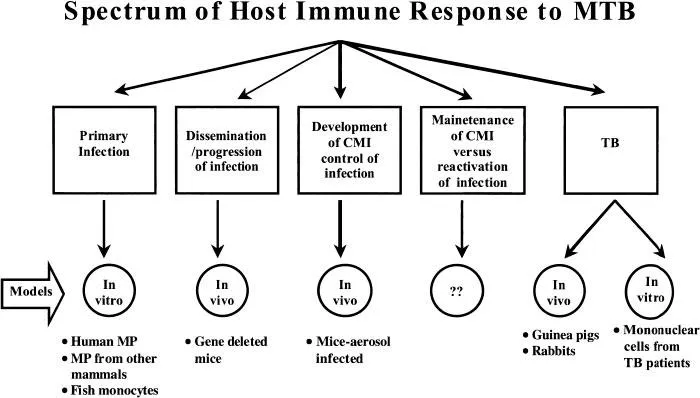
The interaction of the host immune response with MTB can be divided into at least five types of events. 1. Events of primary MTB infection. 2. Events that are conducive to dissemination/progression of a primary infection. 3. Development of specific immunity that leads to containment of infection. 4. Maintenance of protective immunity versus immunologic events related to reactivation of MTB infection. 5. Immunologic events associated with active disease and immunopathology of TB. It is important to understand that involvement of components of the host immune system such as mononuclear phagocytes varies during the spectrum of MTB infection. Moreover, monocytes emigrate from the bone marrow and then differentiate to tissue macrophages in unique fashion in different organs. Thus, the molecular mechanisms underlying phagocytosis and intracellular growth of MTB in macrophages likely differ depending on the tissue site, degree of cell differentiation, presence of inflammatory mediators, etc. Various in vitro and in vivo models have been employed to understand these interactions more completely. Each of these models is best suited to assess only one or at most a few stages of the whole spectrum of MTB infection (Figure 1) and therefore any one model is inadequate in providing the full picture. As one example, whereas local production of tumor necrosis factor alpha (TNF-α) may activate macrophages allowing containment and prolonged maintenance of defenses against bacillary growth (Keane et al., 2001), its excess production during active TB contributes to immunopathology (Bekker et al., 2000).
This chapter will focus on the contribution of mononuclear phagocytes to MTB pathogenesis. How macrophages are targeted by bacilli and defeated in the battle against MTB to become safe-havens for intracellular growth will be discussed first. Then, both the in vivo and in vitro models of phagocytosis and growth of MTB within mononuclear phagocytes will be discussed. The focus will be primarily on the events related to primary MTB infection, with some reference to the remainder of the TB spectrum.
The Role of Macrophages in TB Pathogenesis: Phagocytosis and Post-phagocytic Events
Alveolar macrophages (AM) are the primary cell type targeted by MTB, and play a central role in the pathogenesis of this organism. However, the role of other mononuclear phagocytes such as the undifferentiated blood precursors of AM, monocytes (MN), that are heavily recruited to sites of MTB infection (Schwander et al., 1996), and macrophages that have differentiated into dendritic cells (DC) in situ also need to be considered.
MTB enters mononuclear phagocytes by receptor-mediated phagocytosis (Schlesinger et al., 1990) in which several major host cell receptors play a role. These include complement receptors (CRs), the mannose receptor (MR) and type A scavenger receptors (Schlesinger et al., 1990, 1993; Stokes et al., 1993; Hirsch et al., 1994; Zimmerli et al., 1996; Melo et al., 2000). The CRs involved are CRl (CD35) and the leukocyte integrins CR3 (CD11b/CD18) and CR4 (CD11c/CD18) (Harris et al., 2000). The expression of CRs (particularly CR4) and the MR increases during monocyte differentiation into macrophages, and CR4 and the MR are highly expressed on AMs (Speert et al., 1985; Myones et al., 1988). Phagocytosis of MTB by human AMs is greater than that by monocytes and CR4 plays a particularly important role (Hirsch et al., 1994). In the absence of specific antibody, Fcγreceptors do not play a role in phagocytosis (Schlesinger et al., 1990), an important finding since entry via this receptor would be expected to generate a vigorous host response. Phagocytosis of MTB is enhanced in the presence of nonimmune serum as a result of complement component C3 opsonization of bacteria. However, there is evidence for the direct interaction between MTB surface components and CR3 during phagocytosis based on in vitro studies using human and murine macrophages as well as CR3-transfected Chinese hamster ovary (CHO) cell lines (Schlesinger et al., 1993; Stokes et al., 1993; Cywes et al., 1996, 1997).
The macrophage MR is a prototypic pattern recognition receptor (PRR) that binds with high affinity to mannose and fucose-containing glycoconjugates frequently found on the surface of a variety of microbes [reviewed in (Medzhitov et al., 2000)].The MR is a member of a family of C-type lectins that is expressed on MDMs, tissues macrophages, and dendritic cells but not monocytes (Speert et al., 1985;Stahl et al., 1990, 1998). AMs demonstrate high MR activity (Wileman et al., 1986).In contrast to CRs, the macrophage MR mediates phagocytosis of the virulent MTB strains, Erdman and H37Rv, but not the attenuated H37Ra strain (Schlesinger et al., 1993) The MR also mediates uptake of other mycobacteria (Astarie-Dequeker et al., 1999).The linear α1-2-linked oligomannosyl “caps” of MTB cell wall lipoarabinomannan (LAM) serve as ligands for the MR during bacterial phagocytosis (Schlesinger et al., 1994, 1996;Kang et al., 1998).Subtle differences exist in the ability of LAM from different MTB strains to bind to the MR and the inositol phosphatecapped AraLAM from M. smegmatis does not bind to this receptor (Schlesinger et al., 1994). Other MTB surface molecules accessible to the MR are arabinomannans, mannans, and mannoproteins (Ortalo-Magne et al., 1995;Dobos et al., 1996).
The high MR activity on AMs is noteworthy for TB pathogenesis. MR activity is increased by IL-4, IL-13 and glucocorticoids and inhibited by IFN-γ. It has been postulated that induction by these mediators as well as by TGF-β produces an alternative activation state of macrophages with many attributes characteristic of AMs [reviewed in (Goerdt et al., 1999)]. The phenotypic and molecular characteristics of these macrophages differ considerably from those of classically activated macrophages. For example, alternatively activated macrophages express high levels of PRRs such as the MR and scavenger receptors but do not display enhanced killing functions towards microbes (Munder et al., 1998; Becker et al., 2000;Stein et al., 1992). In this regard, the abundant surfactant associated protein, surfactant protein A (SP-A), produced in the lung interacts with macrophages to enhance MR activity (Beharka et al., 2002). In contrast, surfactant protein D (SP-D) blocks the LAM-MR pathway (Ferguson et al., 1999). Furthermore, nitric oxide (NO) and oxidant production in response to stimuli are reduced in these cells (Oren et al., 1963; Fels et al., 1986). There is recent evidence for potential involvement of the MR in mycobacterial infection in humans (Siddiqui et al., 2001; Hill et al., 2001).
Although CRs and the MR are the major receptors that mediate phagocytosis on mononuclear phagocytes, other receptors may also participate in MTB phagocytosis either alone or in conjunction with CRs and/or the MR. CD14 has been found to mediate uptake of nonopsonized MTB by human microglia, the resident macrophage in the brain (Peterson et al., 1995), and uptake of M. bovis by porcine alveolar macrophages (Khanna et al., 1996). Class A scavenger receptors participate in the uptake of nonopsonized M. tuberculosis by MDMs (Zimmerli et al., 1996).Potential MTB ligands for mononuclear phagocytes and nonprofessional phagocytes include a mammalian cell entry protein (Arruda et al., 1993), a heparin-binding hemagglutinin (Menozzi et al., 1998), glucan (Cywes et al., 1997; Ehlers et al., 1998; Schwebach et al., 2002), PE_PGRS proteins (Brennan et al., 2001), phosphatidylinositol mannoside PIM) (Hoppe et al., 1997), and antigen 85 (Hetland et al., 1994).
Recent literature indicates that the nature of receptor-ligand interactions for MTB can regulate the early host cell response and the fate of the bacterium. The C3-CR entry pathway for MTB is postulated to provide the bacterium safe passage into mononuclear phagocytes. To the potential advantage of the pathogen, ligation of CR3 does not uniformly trigger toxic host cell responses (Wilson et al., 1980) and has recently been shown to selectively suppress interleukin 12 (IL-12) production, an important mediator of the cellular immune response to MTB(Marth et al., 1997). There is little or no oxidative response to MTB by non-activated human macrophages during phagocytosis (Wayne et al., 1995), a result similar to that obtained with M. kansasii using a human myeloid cell line (Le Cabec et al., 2000).
The binding sites for MTB on CR3 may impact on the host cell response and bacterial fate.That there are fundamental differences in the binding interactions to CR3 between C3-opsonized and non-opsonized MTB is supported by in vitro studies in which the phagocyte membrane is depleted of cholesterol to ascertain the importance of cholesterol-rich microdomains (rafts).Cholesterol depletion results in significant reduction in mycobacterial uptake (Gatfield et al., 2000).Cholesterol depletion of neutrophils decreased the uptake of non-opsonic mycobacteria but not serum-opsonized organisms, indicating that nonopsonic uptake via CR3 selectively involves a GPI-anchored protein in membrane rafts (Peyron et al., 2000). Different molecular mechanisms for binding involving distinct epitopes on CR3 lead to transduction of different cellular responses depending on the state of bacterial opsonization (Le Cabec et al., 2002).
The LAM-MR pathway also appears to be preferable for MTB. MR-dependent phagocytosis is not coupled to activation of the NADPH oxidase in nonactivated phagocytes (Ezekowitz et al., 1985; Astarie-Dequeker et al., 1999) and the LAM-MR pathway appears to be important in limiting P-L fusion events (Kang and Schlesinger, 1998;Fratti et al., 2001). MTB LAM is reported to inhibit IL-12 production via the MR by generating a ...