
- 266 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
This introduction to thermodynamics discusses typical phase diagrams features and presents the wide range of techniques such as Differential Scanning Calorimetry, Thermogravimetry and others. In the last part the author brings many examples for typical practical problems often solved by thermal analysis. As an instructive guideline for practitioners the work reveals the connection between experimental data and theoretical model and vice versa.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Thermal Analysis and Thermodynamics by Detlef Klimm in PDF and/or ePUB format, as well as other popular books in Tecnologia e ingegneria & Chimica fisica e teoretica. We have over one million books available in our catalogue for you to explore.
Information
1 Thermodynamic basis
1.1 Specific heat capacity
1.1.1 Phenomenological description
One of the first reports on experiments, which we would now call “thermal analysis”, was given by Le Chatelier (1887),1 who described the heating of different clay minerals. In his experiments, the sample was heated with constant electric power ( and are the voltage and current of the heater) and sample temperature T was recorded by a thermocouple (see Section 1.3.1) at constant time increments. It turned out that the measured heating rate was not constant, because endothermal reactions in the sample (e. g., dehydration processes or phase transitions) resulted in temporally smaller . It will be described in Section 2.1 how such a type of heating (or cooling) experiments was developed further to the modern technique of differential thermal analysis (DTA).
The experimental setup of Le Chatelier corresponds to the scheme in Fig. 1.1. If during time t, the amount of heat energy
(1.1)
is transferred to one mole of a sample, then its temperature increases under isochor (volume constant; ) or the more typical isobar (pressure ; often, ) conditions by
(1.2)
(1.3)
where and are proportionality parameters called the specific heat capacities of the material under the given conditions, V or . Especially for technical purposes, the amount of substance is often given by the mass , where n is the number of moles, and M is the molar mass. The difference
(1.4)
is positive but usually small for condensed phases with small thermal expansion (solids, liquids). For ideal gases, we have (gas constant). Table 1.1 reports some data from the literature (Regen and Brandes, 1979; Paufler, 1986).
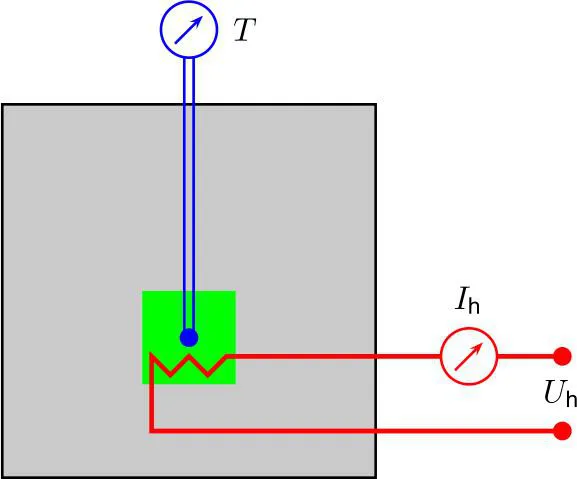
Figure 1.1 The experimental setup used by Le Chatelier (1887) allows the measurement of the specific heat capacity using equation (1.3).
Table 1.1Specific heat capacities near room temperature for several substances. For gases, ideal data are scaled by the gas constant R. Data for solid phases are given in J/(mol K).
| Substance | ||
| 1-atomic gas |
Table of contents
- Title Page
- Copyright
- Contents
- 1 Thermodynamic basis
- 2 Techniques
- 3 Applications
- Subject Index