
Persulfate-based Oxidation Processes in Environmental Remediation
- 350 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Persulfate-based Oxidation Processes in Environmental Remediation
About this book
Advanced oxidation processes (AOPs) use chemical treatment to remove contaminants from water by oxidation with hydroxyl radicals. These hydroxyl radicals can be produced using UV light, ozone or hydrogen peroxide, but recently reactions have been developed that use persulfates as the radical source. Persulfates are strong oxidants with flexible in situ activation characteristics, including activation with heat, alkali conditions, electricity, ultrasonic treatment, transition metals, carbon and even organics. Persulfate activation can generate sulfate radicals as well as other reactive species. These reactive species, especially the sulfate radical, can degrade most organic pollutants making them valuable in the fields of water purification, soil remediation, disinfection, sludge dewatering, and other important applications in environmental systems.
Describing recent developments in persulfate-based AOPs, this book aims to provide a summary of environmental applications for persulfate-based AOPs and to guide the reader, in a comprehensive way, through various advanced oxidation processes in environmental applications. Topics include new activation methods, activation mechanisms, and advanced materials for use in activating persulfate-based AOPs for different environmental applications.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1.1 Introduction
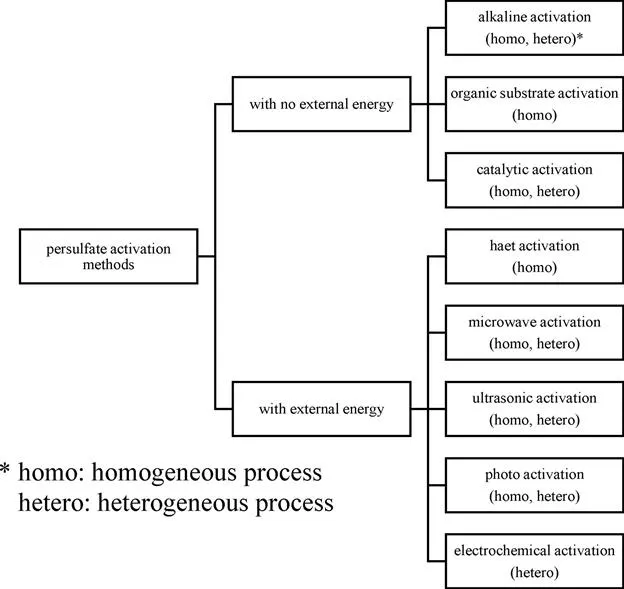
1.2 Alkaline Activation
1.2.1 Basic Concepts
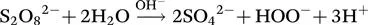
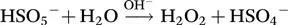
1.2.2 Application of a Catalyst
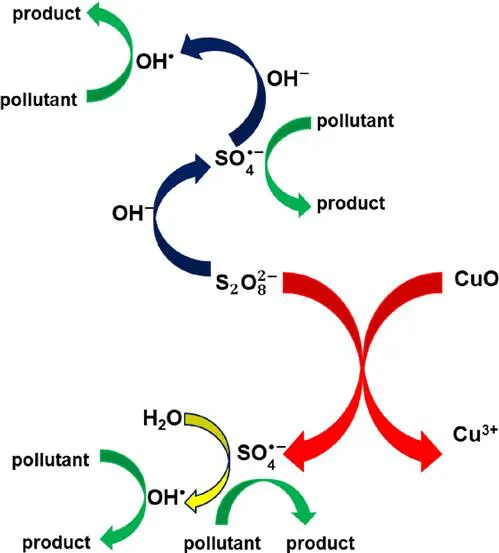
1.2.3 Influence of Operating Conditions
1.2.3.1 Persulfate Concentration
Table of contents
- Cover
- Title
- Copyright
- Contents
- Chapter 1 Methods of Persulfate Activation for the Degradation of Pollutants: Fundamentals and Influencing Parameters
- Chapter 2 Photo-activated Persulfate-based Advanced Oxidation for Water Treatment
- Chapter 3 Electrochemical Activation of Persulfate for Organic Pollution Control in Water
- Chapter 4 Reactive Oxygen Species in Catalytically Activated Peroxydisulfate
- Chapter 5 Heterogeneous Activation of Persulfate Using Metal and Metal Oxides
- Chapter 6 Metal-free Carbocatalysis for Persulfate Activation Toward Organic Oxidation
- Chapter 7 Persulfate-based Advanced Oxidation Processes in Environmental Remediation: Theoretical Chemistry Study
- Chapter 8 Sulfate Radical-based Advanced Oxidation Processes for Water and Wastewater Disinfection
- Chapter 9 Inactivation of Pathogenic Microorganisms with Sulfate Radical-based Advanced Oxidation Processes
- Chapter 10 Persulfate Application for Landfill Leachate Treatment: Current Status and Challenges
- Chapter 11 Novel Strategy for Soil Remediation of Contaminated Sites Using Persulfate-based Advanced Oxidation Technologies
- Chapter 12 Persulfate-based Advanced Oxidation Processes in Other Applications
- Subject Index