
- 228 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
This book describes more than thirty physics practicals at high school and undergraduate levels with background information on each one, a description of the equipment needed, and instructions on how the experiment is performed. Uniquely, for those without access to a real laboratory, the book provides access to highly detailed 3D simulations of all the experiments.
The simulations are a superset of the Virtual Physics Laboratory as reviewed and given the Green Tick of Approval by the Association for Science Education. They run in any browser that supports WebGL, such as Microsoft Edge or Firefox on Windows and Safari on Mac. For the school or university student who wants to practice and widen their knowledge of physics, or for those who are learning on their own, this is an ideal book for honing and broadening experimental skills.
The simulations are the result of many years researching the teaching of online science, a field in which the author has published many papers.
The companion website for the book can be found here: https://www.virtual-science.co.uk/
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1 Millikan’s Oil Drop Experiment
Introduction
- Question: What is the volume of a single polymer ball?
- Question: What is the mass of a single polymer ball given that the density is 1.05 g/ml?
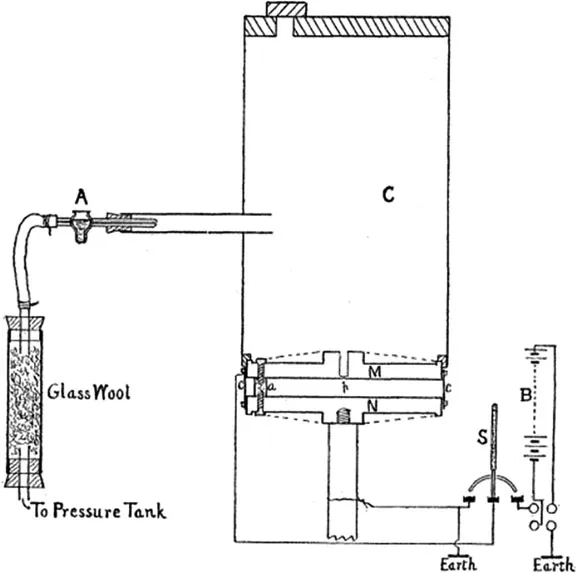
The Objective
The Apparatus

- A high-voltage power supply for the plates with polarity and voltage control
- A low-voltage power supply for the light bulb
- An electrostatic chamber
- A multimeter
- A bottle of polymer balls
- A squeeze atomizer
- A light source
- A telescope or webcam with reticle
- A micrometer
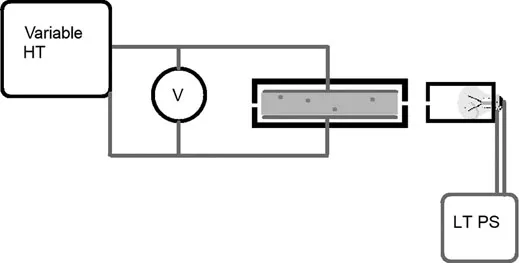
The Variables
The Physics
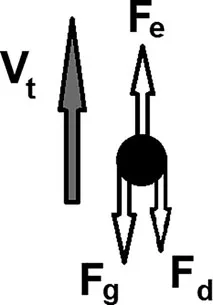
Fe = Fg + Fd
Fe = qE
Table of contents
- Cover
- Half Title
- Title
- Copyright
- Dedication
- Contents
- About the Author
- Introduction
- 1 Millikan’s Oil Drop Experiment
- 2 Planck’s Constant
- 3 Rutherford’s Gold Foil Experiment
- 4 Measuring the Acceleration Due to Gravity
- 5 Average Velocity Using an AirTrack
- 6 Determining Acceleration Using an AirTrack
- 7 Confirmation of Newton’s Second Law
- 8 Showing Conservation of Energy Using an AirTrack
- 9 Conservation of Momentum in an Inelastic Collision Using an AirTrack
- 10 Hooke’s Law
- 11 Young’s Modulus
- 12 Velocity of Rifle Shell Using a Ballistic Balance
- 13 Simple Pendulum
- 14 Simple Harmonic Motion Using a Mass-Spring System
- 15 Capacitor Charge and Discharge
- 16 The Internal Resistance of a Dry Cell
- 17 The IV Characteristics of a Diode
- 18 The IV Characteristics of a Filament Lightbulb
- 19 The Resistivity of Constantan
- 20 Resistors in Series and Parallel
- 21 Heat Transfer
- 22 Boyle’s Law
- 23 Charles’s Law
- 24 Mechanical Equivalent of Heat
- 25 Specific Heat Capacity of Brass
- 26 Investigation of Mechanical Waves
- 27 Measuring the Speed of Water Ripples
- 28 Infrared Radiation
- 29 Diffraction Using a Monochromatic Laser
- 30 Inverse Square Law for Gamma Radiation
- 31 Refraction of Light
- 32 Magnetic Field Due to a Coil of Wire
- 33 Investigation of Magnetic Flux of a Current-Carrying Wire
- 34 Magnetic Flux Linkage
- Appendix 1 Uncertainties
- Appendix 2 Using Excel for the Results
- Appendix 3 Controlling the Simulations
- Index