
Medical Devices and IVDs
Fit for the new EU-Regulations: Your complete seminar for projekt, study and job
- 344 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Medical Devices and IVDs
Fit for the new EU-Regulations: Your complete seminar for projekt, study and job
About this book
With this book, you get a really complete seminar for the new Regulations on medical devices and IVDs in the EU, ready at hand, at any time.These EU regulations create new rules for medical technology and laboratory diagnostics in Europe. Concise regulatory know-how is now required to keep or reposition medical devices and in vitro diagnostics on the European market, from syringes, contact lenses, medical device apps, pregnancy tests, nuclear magnetic resonance tomography to cancer tests, genetic diagnostics, HIV tests, hip implants, heart catheters, artificial spinal discs, stents and pacemakers. Concise regulatory training and further education of employees in companies and health care facilities is the order of the day. This also applies to biomedical and medical technology students at universities of applied sciences and biomedical universities, start-ups and spin-offs, who must make use of this know-how from the initial product idea through the further stages of product development to market access.The book provides a thorough, compact course on the new regulations, starting with perfect overview and easy navigation and going into depth where you need it: this book will make you fit and confident for the new European challenges!344 pages; 47 col. figures; 26 tables
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Chapter 1. Overview of the New Regulatory System for Medical Devices and In Vitro Diagnostics in the EU
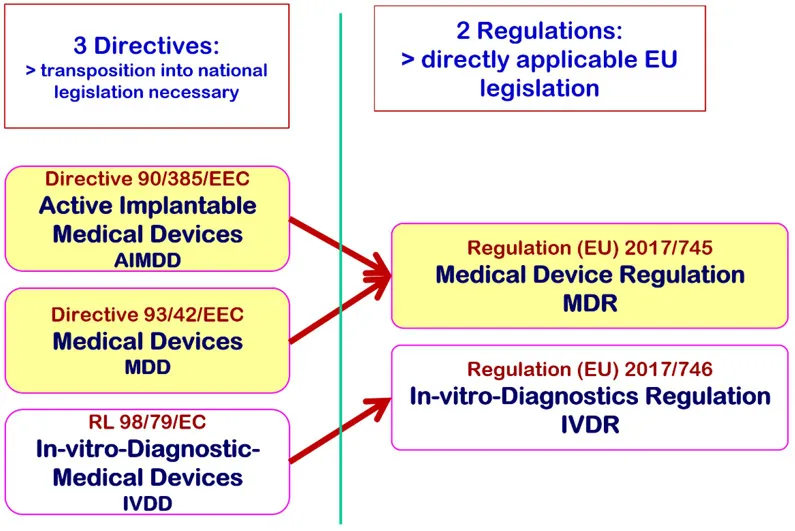
1.1. Transition from Directives to Regulations
- Recitals, i.e. intentions and objectives of the EU legislator which can be used as an aid to interpretation in the event of legal uncertainties,
- the Chapters and their Articles, with the core of both legal texts, and
- the Annexes, the binding, more technically oriented legal text, which supplement the chapters (for example Annex I on General Safety and Performance Requirements [GSPR; Annex II and III on technical documentation or Annexes IX to XI on conformity assessment modules [= modules of "European Premarket Approval"]).
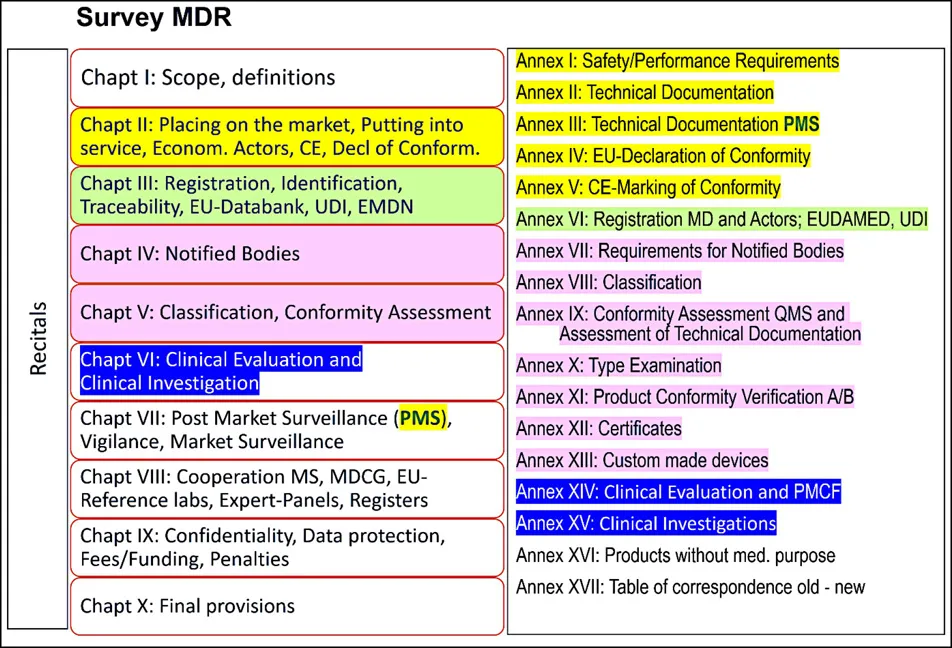
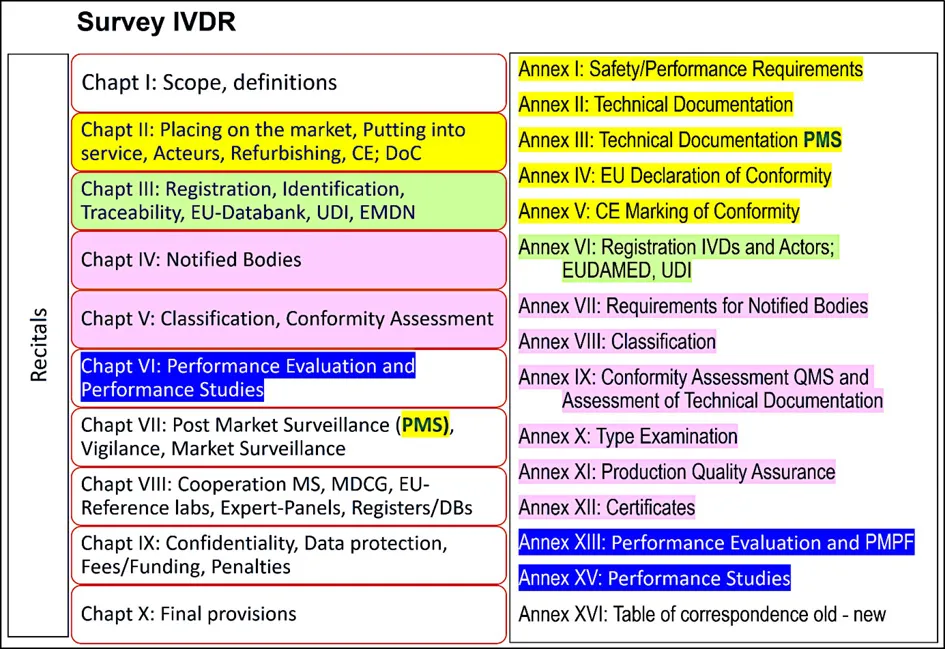
1.2. The New EU Regulatory System for Medical Devices and IVDs: The Building Principles
1.2.1. New legal framework for EU product legislation
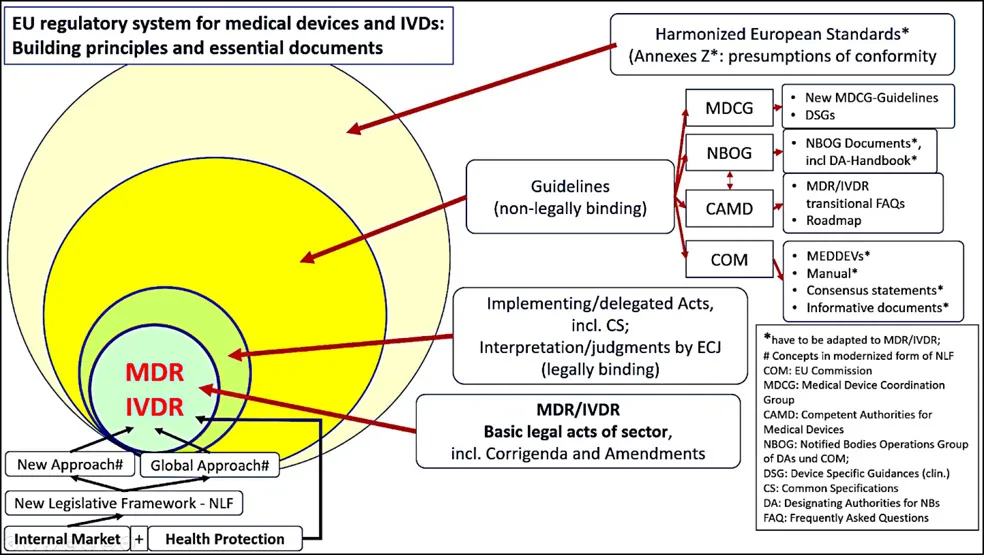
- New Approach and the
- Global Approach.
1.2.2. The New Approach Under the New Legal Framework
Table of contents
- Table of Contents
- Preface
- Acknowledgement
- Chapter 1. Overview of the New Regulatory System for Medical Devices and In Vitro Diagnostics in the EU
- Chapter 2. The Scope of the Two New Regulations: Product Delineation as a MD/IVD (Qualification)
- Chapter 3: General Obligations of Economic Operators
- Chapter 4. European Databank EUDAMED and its Modules; UDI-System; Identification and Traceability of MD/IVD; European Medical Device Nomenclature (EMDN)
- Chapter 5. General Safety and Performance Requirements for Medical Devices and IVDs
- Chapter 6. Clinical evaluation of medical devices
- Chapter 7. Performance Evaluation of In Vitro Diagnostics
- Chapter 8. Clinical investigation of medical devices
- Chapter 9. Performance studies of IVDs
- Chapter 10. Technical Documentation (TD)
- Chapter 11. Classification
- Chapter 12. Conformity Assessment of MDs
- Chapter 13. Conformity Assessment of IVDs
- Chapter 14. Surveillance After the Start of Placing on the Market, Responsibilities
- Chapter 15. Post-Market Surveillance (PMS), PMS System
- Chapter 16: The Vigilance System
- Chapter 17. Service Part
- Copyright