
- 144 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Written by an experienced professional, this book introduces chemists to process development, using examples from the pharmaceutical, agrochemical and fragrance industries. The focus is on small molecules rather than biomolecules, and on relatively small-scale production rather than bulk petrochemicals. The coverage is broad, going from initial route development, through pilot plant operations, to full-scale production.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1 Route, reagent and solvent selection
1.1 Basic route selection
There are always a huge number of ways in which you could conceivably make any reasonably complex molecule, and the methods that have been used previously are not necessarily the most efficient. Certain guidelines are useful in planning a synthesis. Firstly, we can consider the two synthetic routes below, both forming the final product D:
A → B → C → D
and
E → F; G → H; F + H → D
Both reaction schemes consist of three reactions. If each step has a molar yield of 90%, the top linear reaction scheme would give an overall molar yield of 72.9%, while the lower convergent synthesis would give an overall molar yield of 81.0%. Usually, it is best to disconnect larger molecules into two or three roughly equal portions in order to maximise yields, rather than continually extend a starting material a little at a time.
All syntheses must start from commercially available starting materials, the cheaper the better. A knowledge of which molecules are relatively inexpensive is very useful. For chiral products, it is often beneficial to select starting materials that have the correct chirality ‘built in’ – the so-called chiral pool.
The obvious basic building blocks are the starting materials for many syntheses, but other starting materials are less evident. For example, furfural (Fig. 1.1), which is isolated from farm waste such as corn cobs and oat husks, is a relatively inexpensive starting material.
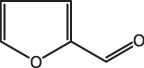
Fig. 1.1: Furfural, a useful starting material.
However, even the putting together of ‘simple’ building blocks may not always be straightforward. Consider the formation of a ‘mesityl oxide portion’ of a larger molecule, which might be required as an intermediate for a pharmaceutical or fragrance compound. Here the obvious synthetic method (Fig. 1.2) is the aldol condensation of a methyl ketone (a) with acetone, to give the hydroxyketone (b), which readily dehydrates with acid to give the mesityl oxide analogue (c). However, simply reacting (a) with acetone in the presence of a base gives a complex mixture of products, since both compound (a) and acetone can react with themselves and also react with each other in more than one way, giving large quantities of various unwanted condensation products. The literature [1] suggests the reaction is carried out using lithium diisopropylamide (LDA) as base at low temperatures, giving the kinetic enolate of (a), and then slowly adding acetone. Such a procedure gives good yields of (b), but is very expensive to implement on a plant scale, where temperatures below those obtained with chilled ethylene glycol (around –15 to –20 °C in practice) are difficult to reach in standard vessels. Also, LDA, though available in solution in bulk metal drums, is a relatively expensive reagent to use.
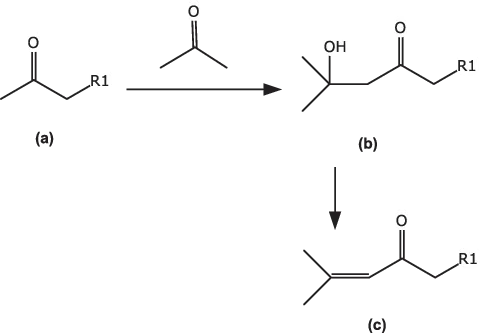
Fig. 1.2: Aldol condensation of a methyl ketone with acetone.
A totally different approach would be a Friedel-Crafts acylation (Fig. 1.3) between the acid chloride (d) and isobutylene in the presence of a Lewis acid, to give a mixture of (c) and the chloride (e), which readily loses HCl in the presence of a base to give (c). Isobutylene has a boiling point of about –7 °C and is a useful reactant in Friedel-Crafts reactions with acid chlorides [2], which can be carried out at –10 to –20 °C. Isobutylene does form dimers to some extent, but these octene compounds are readily distilled out, unlike acetone condensation products. Such reactions might require a stoichiometric amount of a Lewis acid (TiCl4 or AlCl3 are likely candidates) and would require a solvent that is compatible with such acids. Dichloromethane is an obvious choice, but chlorinated solvents are frowned upon nowadays for environmental reasons.
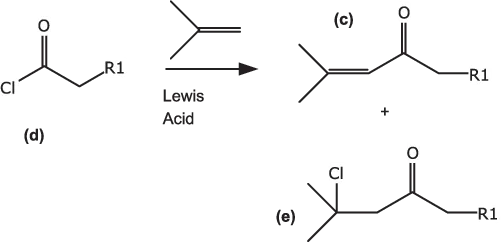
Fig. 1.3: Acylation of isobutylene.
Other routes to (c) involving metal coupling reactions or via various enol compounds are also possible, but the point is that even a ‘simple’ transformation may not be straightforward on a plant scale, and careful consideration of the likely capital, raw material and running costs are needed, along with an examination of the environmental and safety factors, in order to choose the best route for the process.
1.2 Syntheses from chiral starting materials
A number of naturally occurring terpenoid compounds are inexpensive, and some act as a useful source of chirality. For example, (R)-limonene (Fig. 1.4) is readily available from orange peel, etc.
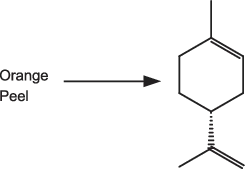
Fig. 1.4: Limonene, a useful chiral starting material.
(R)-Limonene (Fig. 1.5, f) has been used in cannabinoid syntheses. Conversion to the key intermediate p-mentha-2,8-dien-1-ol (g) was initially accomplished using a rose-bengal photosensitiser to generate singlet oxygen [3], but the use of the latter species is challenging on a large scale. However, a recent paper [4] described a version of this reaction using tetraphenylporphyrin, rather than rose bengal, as photosensitiser in a continuous reactor. A selectivity of 66% was achieved with 55% conversion and an output of around 66 g/day. Further development might lead to a commercial process.
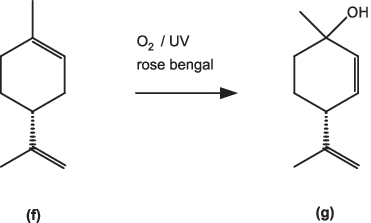
Fig. 1.5: Preparation of p-mentha-2,8-dien-1-ol (g) using singlet oxygen.
A longer synthesis (Fig. 1.6) was devised by Firmenich chemists [5], avoiding the use of singlet oxygen. Initial oxidation of (R)-limonene (f) gave the epoxide (h), which was selectively reacted with thiophenol to give the sulfide (i). Hydrogen peroxide gave the sulfoxide (j), which underwent elimination to (g) on heating at 425 °C.
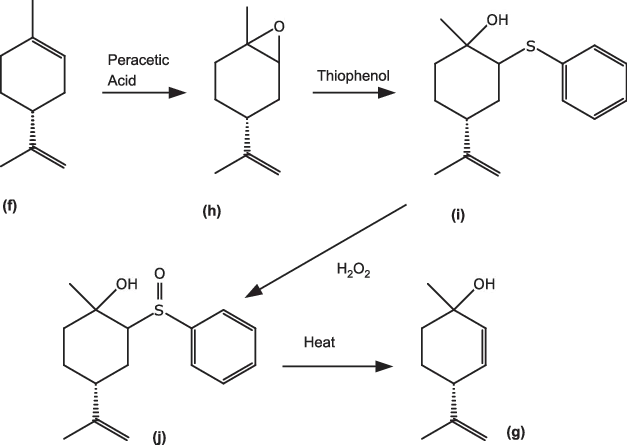
Fig. 1.6: Preparation of p-mentha-2,8-dien-1-ol (g) via sulfoxide elimination.
Reaction of the intermediate p-mentha-2,8-dien-1-ol (Fig. 1.7, g) with olivetol (k) gives a mixture of different cannabinoids, the predominant species present depending on the conditions [3] employed. Mild acids give mainly cannabidiol (CBD, l), stronger acids, such as p-toluene sulfonic acid (p-TSA), give mainly ∆8-THC (m), while reacting with boron trifluoride and magnesium sulphate in dichloromethane at 0 °C gives mainly ∆9-THC (n), the psychoactive constituent of smoked marijuana.
...
Table of contents
- Title Page
- Copyright
- Contents
- Introduction
- Chapter 1 Route, reagent and solvent selection
- Chapter 2 Scale-up hazards
- Chapter 3 Purification
- Chapter 4 Polymorphs
- Chapter 5 Optimisation and experimental design
- Chapter 6 Automation
- Chapter 7 Environmental and toxicology issues
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Process Development by Jerry Carr-Brion in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Industrial & Technical Chemistry. We have over one million books available in our catalogue for you to explore.