![]() Evolutionary Perspectives on Nutrition
Evolutionary Perspectives on Nutrition![]()
• 1 •
What Did Humans Evolve to Eat?
Metabolic Implications of Major Trends in Hominid Evolution
W. R. Leonard, M. L. Robertson, and J. J. Snodgrass
Introduction
Over the last twenty years, the evolution of human nutritional requirements has received ever-greater attention among both anthropologists and nutritional scientists (Crawford 1992; Eaton and Konner 1985; Garn and Leonard 1989; Leonard and Robertson 1992, 1994; Aiello and Wheeler 1995; Cordain et al. 2005; Ungar 2007). Research in nutritional anthropology has demonstrated that many of the key features that distinguish humans from other primates have important implications for our distinctive nutritional needs (Leonard 2002; Leonard and Robertson 1997b; Aiello and Wheeler 1995). In addition, our colleagues in the nutritional sciences are coming to realize that an evolutionary perspective is useful for understanding the origins of and potential solutions to the growing problems of obesity and associated metabolic disorders (e.g., Cordain et al. 2005; Eaton 2006; O’Dea 1991).
Yet, despite this growing consensus that an evolutionary approach has an important place in the study of human nutrition, we find that many constructions of the “natural” human diet are remarkably narrow (e.g., Audette and Gilchrist. 2000; Crawford and Marsh 1995; Cunanne 2005; Eaton, Shostack, and Konner 1988). We believe that many of these “paleodiets” are based on a misreading of both human evolutionary history and comparative human biology. Humans did not evolve to subsist on a single Paleolithic diet. To the contrary, one of the hallmarks of our evolutionary success has been our ability to find or create a meal in any environment. Compared to other primates, humans have diets of much higher quality—that is, more dense in calories and nutrients. Indeed, many of the major changes in human evolutionary history have been about increasing the quality of our diets or increasing the efficiency with which we extract energy and nutrients from our environments.
This chapter specifically considers the nutritional implications of one of the most profound transition periods in human evolution—the emergence of the first members of the genus Homo. This phase of human evolution—between ~2.0 and 1.5 million years ago—was associated with major changes in brain size, body size, and foraging and ranging behavior.
To establish the context for interpreting the fossil evidence, we begin by considering the energetic and nutritional correlates of variation in brain and body size among living primates. We then turn to an examination of the human fossil record to consider when and under what conditions in our evolutionary past key changes in brain size, body size, diet, and foraging behavior likely took place. Finally, we explore the implications of our distinctive metabolic requirements for understanding and confronting the nutritional problems of our modern world. We will specifically consider (1) the problem of early childhood growth stunting among populations of the developing world, and (2) the growing problem of obesity in the United States and other industrialized nations.
Comparative Nutrition and Metabolism
From a nutritional perspective, what is extraordinary about our large human brains is their high metabolic costs. Brain tissue has very high energy demands per unit weight, roughly 16 times greater than those of muscle tissue (12 kcal/kg/min versus 0.75 kcal/kg/min; Holliday 1986; Kety 1957). On average about 400 kcal/day are spent on brain metabolism by an adult human. Yet, despite the fact that humans have much larger brains per body weight than other primates or terrestrial mammals, the resting energy demands for the human body are no more than for any other mammal of the same size (Leonard and Robertson 1994).
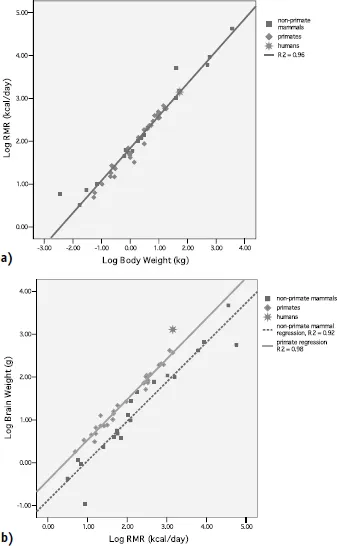
Figure 1.1a shows the relationship between Resting Metabolic Rate (RMR; kcal/day) and body mass in kilograms (kg) for humans and nonhuman primates, and other mammals. It is clear that humans, as well as other primate species, conform to the general mammalian scaling relationship between RMR and body weight, the “Kleiber Relationship” (Kleiber 1961). The Kleiber Relationship shows that metabolic rates in mammals of vastly different sizes increase as a function of body weight raised to the 3/4th power. Thus for a mammal of a given body mass, we can predict their resting energy needs as:
RMR (kcal/day) = 70(Wt0.75)
On average, adult humans have RMRs that fall within 3–4 percent of the value predicted for other primates and other mammals. The implication of this is that humans allocate a much larger share of our daily energy budget for brain metabolism than other species.
The disproportionately higher energy costs of our large brains are evident in the scaling relationship between brain weight (grams) and RMR for humans, thirty-five other primate species, and twenty-two nonprimate mammalian species (Fig. 1.1b). The solid line denotes the best-fit regression for nonhuman primate species, and the dashed line denotes the best-fit regression for the nonprimate mammals. The data point for humans is denoted with a star.
Figure 1.1. a) Log-Log plot of resting metabolic rate (RMR; kcal/day) versus body weight (kg) for 51 species of terrestrial mammals (20 non-primate mammals, 30 primates, and humans). Humans conform to the general mammalian scaling relationship, as described by Kleiber (1961); b) Log-Log plot of brain weight (BW;g) versus RMR (kcal/day) for humans, 35 other primate species, and 22 non-primate mammalian species. The primate regression line is systematically and significantly elevated above the non-primate mammal regression. For a given RMR, primates have brain sizes that are three times those of other mammals, and humans have brains that are three times those of other primates.
As a group, primates have brains that are approximately 3 times the size of other mammals (relative to body size). Human brain sizes, in turn, are some 2.5 to 3 times those of other primates (Martin 1989). In caloric terms, this means that brain metabolism accounts for ~20–25 percent of RMR in an adult human body, as compared to about 8–10 percent in other primate species, and roughly 3–5 percent for nonprimate mammals (Leonard et al. 2003).
The large allocation of our energy budget to brain metabolism raises the question of how humans are nutritionally able to accommodate the metabolic demands of our large brains. It appears that humans consume diets that are more dense in energy and nutrients than other primates of similar size.
Across all primates, diet quality is inversely related to body size. That is, small primates (e.g., the pygmy marmoset) consume diets that are rich in energy and nutrients, whereas large-bodied primates (e.g., the gorilla) consume large amounts of low-quality foods (Richard 1985). These feeding strategies are shaped by between-species variation in metabolic rates, specifically the Kleiber Relationship, mentioned previously.
Small-bodied primates have low total energy needs but very high energy demands per unit mass (i.e., kcal/kg/day). Consequently, they meet their dietary needs by consuming foods that are limited in abundance but high in quality (insects, saps, gums). Large primates have high total energy need, but low mass-specific costs. Hence they are large-volume feeders, eating foods that are widely available, but of low nutritional density (leaves, bark, and low-quality plant foods).
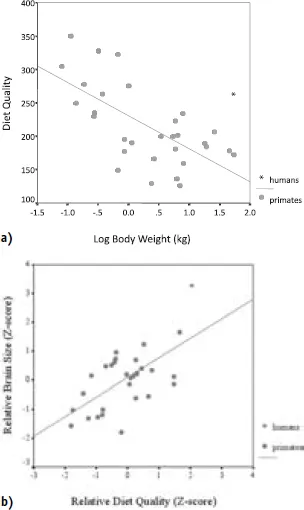
Humans, however, have substantially higher-quality diets than expected for a primate of our size. Figure 1.2 shows the association between dietary quality and body weight in living primates, including modern human foragers. The diet quality (DQ) index is derived from the work of Sailer et al. (1985) and reflects the relative proportions (percentage by volume) of (1) structural plant parts (s; e.g., leaves, stems, bark), (2) reproductive plant parts (r; e.g., fruits, flowers), and (3) animal foods (a; including invertebrates):
DQ index = s + 2(r) + 3.5 (a)
The index ranges from a minimum of 100 (a diet of all leaves and/or structural plant parts) to 350 (a diet of all animal material).
There is a strong inverse relationship between DQ and body mass across primates; however, note that the diets of modern human foragers fall substantially above the regression line in Figure 1.2a. Indeed, the staple foods for all human societies are much more nutritionally dense than those of other large-bodied primates. Although there is considerable variation in the diets of modern human foraging groups, recent studies have shown that modern human foragers typically derive over half of their dietary energy intake from animal foods (Cordain et al. 2000). In comparison, modern great apes obtain much of their diet from low-quality plant foods. Gorillas derive over 80 percent of their diet from fibrous foods such as leaves and bark (Richard 1985). Even among common chimpanzees (Pan troglodytes), only about 5–10 percent of their calories are derived from vertebrate animal foods (Teleki 1981; Stanford 1996). This “higher-quality” diet means that we need to eat a lower volume of food to get the energy and nutrients we require.
The link between brain size and dietary quality is evident in Figure 1.2b, which shows relative brain size versus relative dietary quality for the thirty-three different primate species for which we have metabolic, brain size, and dietary data. Relative brain size for each species is measured as the standardized residual (z-score) from the primate brain versus body mass regression, and relative DQ is measured as the residual from the DQ versus body mass regression. There is a strong positive relationship (r = 0.63; P < 0.001) between the amount of energy allocated to the brain and the caloric and nutrient density of the diet. Across all primates, larger brains require higher-quality diets. Humans fall at the positive extremes for both parameters, having the largest relative brain size and the highest quality diet.
Figure 1.2. a) Plot of diet quality (DQ) versus log-body mass for 33 primate species. DQ is inversely related to body mass (r = -0.59 [total sample]; -0.68 [non-human primates only]; P < 0.001), indicating that smaller primates consume relatively higher quality diets. Humans have systematically higher quality diets than predicted for their size; b) Plot of relative brain size versus relative diet quality for 31 primate species (including humans). Primates with higher quality diets for their size have relatively larger brain size (r = 0.63; P < 0.001). Humans represent the positive extremes for both measures, having large brain:body size and a substantially higher quality diet than expected for their size.
Thus, the high cost of the large, metabolically expensive human brain is partially off set by the consumption of an energy- and nutrient-rich diet. This relationship implies that the evolution of larger hominid brains would have necessitated the adoption of a sufficiently high-quality diet (including meat and energy-rich fruits) to support the increased metabolic demands of greater encephalization.
Evolutionary Trends in Diet, Brain Size, and Body Size
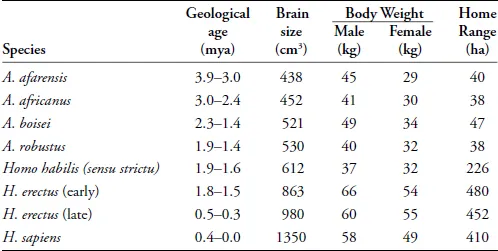
When we look at the human fossil record, we find that the first major burst of evolutionary change in hominid brain size occurs at about 2.0 to 1.7 million years ago, associated with the emergence and evolution of early members of the genus Homo (see Table 1.1). Prior to this, our earlier hominid ancestors, the australopithecines, showed only modest brain size evolution from an average of 400 to 510 cm3 over a 2-million-year span from 4 to 2 million years ago. With the evolution of the genus Homo there is rapid change, with brain sizes of, on average, ~600 cm3 in Homo habilis (at 2.4–1.6 mya) and 800–900 cm3 in early members of Homo erectus (at 1.8–1.5 mya). Furthermore, while the relative brain size of Homo erectus did not reach the size of modern humans, it is outside of the range seen among other living primate species.
Table 1.1. Geological ages (millions of years ago), brain size (cm3), estimated male and female body weights (kg), and estimated home range sizes (hectares) for selected fossil hominid species.
Data for brain size and body weights are from McHenry and Coffing (2000), except for Homo erectus. Early H. erectus brain size is the average of African specimens as presented in McHenry (1994b), Indonesian specimens from Antón and Swisher (2001) and Georgian specimens from Gabunia et al....