![]()
BOOK III
CAPTURING CARBON
Progress! You politicians are always talking about it. As though it were going to last. Indefinitely. More motors, more babies, more food, more advertising, more money, more everything, forever. You ought to take a few lessons in my subject. Physical biology. Progress indeed! What do you propose to do about phosphorus, for example?
— Lord Edward Tantamount
in Aldous Huxley’s Point Counter Point (1928)
Huxley’s point about phosphorus, which he also made in Brave New World (1932) with a Ministry of Crematoria and Phosphorus Reclamation, is that we live on a finite planet and yet we scatter life-giving minerals to the oceans and the winds as if they were infinite. Percentages of nitrogen, phosphorus, and potassium are prominently displayed on every package of fertilizer, and arguably our ability to obtain nitrogen from the air and mine phosphorus and potassium enabled us to reach a population of seven billion. Conventional agriculture likely has enough potassium to last several centuries, but, as Huxley warned, phosphorus is a different story. The carbon and nitrogen cycles, as we shall see, are even more stressed, more out of balance, and more population-threatening.
What Huxley’s Lord Tantamount was chiding politicians about is their stalwart devotion to abstractions like economic growth or progress while turning a blind eye to the needs of natural processes that support all human activity. Whether we chart the phosphorus cycle, mountain snows, or fish populations in coral reefs, the trends of the past decades are ominous and alarming. Whatever we are doing, it is not progress.
![]()
15
Carbon Farming
AS CONVENTIONAL FARMING SPREAD FROM SUMERIA to the rest of the world, the soil biology deficit steadily grew. Measured in carbon, we can put this deficit in 2010 at 30 to 75 percent, depending on location, pre-existing soil type, climate, terrain, drainage, and land use or abuse.
The good news is that solely by changing our farming practices to ones more like the ancients we can take carbon out of our air while, on average, incorporating a layer of carbon into our soil equal to 0.081 inches (2.1 millimeters) over the 8.5 percent of the area of the Earth that we currently use to make food. While degraded soils are the most receptive carbon sink, nearly all soils can be improved by adding carbon.
The process of restoring the soil’s biological life, which we’ll call carbon farming, not only draws carbon from the atmosphere to the soil, it also increases biomass productivity, increases food production, improves nutritional value, helps water purification, reduces energy and fertilizer requirements, controls pests and weeds, and significantly increases biodiversity.
Many of the soils of the world — in the American plains, the Australian outback, the Ural foothills, and the Fertile Crescent — once had carbon content of up to 20 percent, before the advent of the plow and goat. They are typically at 0.5 to 5 percent today. Because fertility varies, it is possible that we can get more return for our carbon investment in some places than others. Generally, the more “worn out” the soil, the more carbon it can take back. This is good news for Africa, Australia, and other areas of depleted soils.
Soil scientist Rattan Lal of Ohio State University found that with better carbon management practices, soils in the continental US could soak up 330 million tons of carbon each year, enough to more than offset the emissions from all the cars in the US, while improving food production by 12 percent.1 Lal says the ultimate potential for soil carbon uptake is one billion tons — a gigaton — per year (1 GtC/yr).2 This contrasts with the 800 gigatons of carbon in the atmosphere, about half of that being anthropogenic. Lal’s estimate may be low, but if it were accurate, and if man-made emissions could be brought down to zero, carbon farming could cut carbon in the atmosphere by one part per million every four years. We are currently raising it by nearly two parts every year, so cutting emissions is still the key to returning to a safe climate.
We have no shortage of carbon to draw upon. The atmosphere carbon pool increased by 3.3 GtC/yr during the 1980s, 3.2 GtC/yr during the 1990s, and an average of 4.1 GtC/yr between 2000 and 2005. The concentration of carbon dioxide has increased 39 percent from the pre-industrial level of 280 ppmv to 390 ppmv today. We can reverse that process using biochar in combination with carbon farming and tree planting. Few other human activities can make that claim.
The uptake of CO2 by natural sinks (terrestrial biosphere and the ocean) has been about 50 percent of total emissions each year, which is to say that Gaia, the geo-chemico-biological stasis of the planet, has been able to absorb half of what we gave her, but no more. Of that, the uptake by the terrestrial biosphere (soils and trees) was about 1 gigaton of carbon per year higher in the 1990s than during the 1980s, which indicates that Gaia has been stretching a little to accommodate our higher atmospheric emissions. However there are signs that she has reached her limits.
Calculating Carbon
One gigaton is a billion tons. An English ton — 2000 pounds — is 10 percent less than a metric ton — 1000 kilograms. For simplicity, I use them interchangeably.
The molecular weight of carbon dioxide (C = 12 + O = 16 + O =16) is 44. Carbon alone is 12. Therefore the molecular weight of Carbon Dioxide Equivalent (CO2e) is calculated at 3.67 times the weight of carbon alone.
The total amount of organic carbon in all of Earth’s soils is estimated to be 3200 GtC, which constitutes about 70 percent of the carbon in terrestrial ecosystems.3 The carbon dioxide stored in plants, 650 GtC, is 81 times all annual man-made CO2 emissions (8 GtC). Thus, diverting only a small proportion of this plant growth to cycles that would hold carbon out of the atmosphere — in “farmed carbon,’ trees, houses, bamboo furniture, wooden ships, or biochar — would begin arresting climate change.
As contrasted with the crystalline, recalcitrant carbon in biochar, the usual form of carbon molecules found in biological systems is called “labile” because it is very active and easily attaches to other organic molecular chains in solid, liquid, and gaseous forms. Plants and animals, ourselves included, need labile carbon to form the building blocks of cellular tissue, including the DNA nucleoprotein helices in all living things.
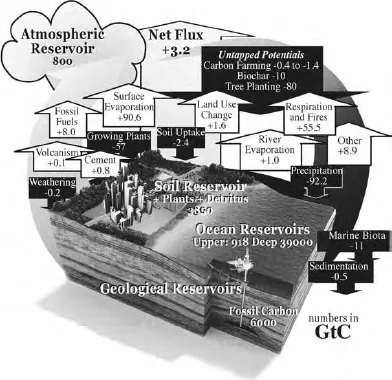
Fig. 10: The carbon cycle. “GtC” represents a gigaton (one billion tons) of carbon.
There is a big difference in the amount of carbon sequestration realized from recalcitrant versus labile carbon management practices. Recalcitrant carbon sequesters 25 to 50 percent of the carbon of its source feedstock for 1000 years. By comparison, making labile carbon by biological decomposition (plowing under) or torrifaction (stubble-burning) sequesters less than 10 to 20 percent for 5 to 10 years.
Johannes Lehmann of Cornell University looked at how fast the greenhouse balance could be shifted using biochar. Depending on various assumptions of how it was made and applied, he estimated the global storage capacity at 224 GtC for cropland and 175 GtC for temperate grasslands; he did not examine forests. Said Lehmann, “These total sequestration opportunities are high and approach levels for total C in plants, ” meaning that the soil uptake potential for biochar could be as high as the total weight of all the biomass on Earth. Lehmann estimates 12 percent of total fossil fuel emissions could be offset annually if slash-and-burn techniques in tropical cultures were replaced by slash-and-char (burying the smoldering biomass during and after the burn), and a comparable amount could be offset by diverting wastes such as forest residues, mill residues, field crop residues, or urban garbage, to make biochar.4
The Carbon Balance
The present atmospheric concentrations of greenhouse gases are 800 gigatons of carbon (GtC), 3000 gigatons of carbon dioxide (GtCO2), or 3300 gigatons of carbon dioxide and equivalent gases (GtCO2e). Each year, human activities add more than 8 GtC, 30 GtCO2, or 33 GtCO2e.
The annual air-earth-air carbon cycle moves 58 GtC or 215 GtCO2. Of cyclical carbon stores, 21 percent is in plants and marine biota (600 GtC), 27 percent is in the atmosphere (800 GtC), and 52 percent is in the soil (1500 GtC).
The oceans hold another 40,000 GtC in dissolved carbonates that normally do not cycle very much — at cold temperatures. But as the oceans warm, the carbon will begin to return to the atmosphere as CO2 and methane (CH4). Presently the oceans absorb 3 GtC/yr, but that is declining.
Atmospheric components are also defined in parts per million by volume. The threshold at which Earth shifts to a 5-degree-warmer equilibrium is considered to be 95 ppmv C, 350 ppmv CO2, or 393 ppmv CO2e. At present the atmosphere contains 100 ppmv C, 390 ppmv CO2, or 436 ppmv CO2e.
It takes 2.12 GtC to add 1 ppmv CO2. At present, atmospheric carbon grows by 4.5 GtC/yr from human activity, resulting in a 2-ppmv increase each year.
Carbon farming on degraded soils involves conversion from plow tillage to organic no-till farming, with crop residue mulch and cover cropping, integrated nutrient management and manuring, and complexing of cropping with rotational grazing and agroforestry.
A farm that switches to organic no-till can sequester 1 to 4 tons of organic matter per acre per year. By employing perennial polycultures, rotated pastures of grazing animals, trees, and wild plant strips, that amount can be doubled or tripled. Plantings of appropriate species, stand management, and polyculture guilding can be applied to woodlots and forests to bring them into the carbon farming regime.
Types of Soil Carbon
Soil uptake of carbon dioxide falls into two distinct categories: soil organic carbon (SOC) and soil inorganic carbon (SIC). Carbon sequestration begins with photosynthesis, which inhales CO2 to create biomass. Part of that biomass is processed into SOC by root hairs left in the ground and by microbes that die and leave their remains. The SOC quickly degrades through physical, chemical, and biological processes, and much of it eventually off-gases back to the atmosphere. Increasing the SOC pool size has a positive impact on net carbon in the soil, and on stabilization of soil structure. It increases water and nutrient retention and species diversity within the soil, and it speeds the recycling of nutrients.
The sequestration of SIC occurs when CO2 becomes carbonic acid and precipitates carbonates of calcium and magnesium. Leaching of bio-carbonates into the gro...