
- 404 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
The book comprises nine chapters, with seven core chapters dealing in detail with the basic principles and processes of the main hydrological components of the water cycle: precipitation, interception, evaporation, soil water, groundwater, streamflow and water quality. It takes a broadly non-mathematical approach, although some numeracy is assumed particularly in the treatment of evaporation and soil water. The introductory and concluding chapters show the relations and interactions between these components, and also put the importance of water into a wider human context – its significant role in human history, its key role today, and potential role in future in the light of climate change and increasing global population pressures. The book is thoroughly up-to-date, contains over 100 diagrams and photographs to explain and amplify the concepts described, and contains over 750 references for further study.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
© IWA Publishing 2017. Mark Robinson and Roy Ward. Hydrology: Principles and Processes. DOI: 10.2166/9781780407296_008
Introduction
“There is no life without water. It is a treasure indispensable to all human activity.”
European Water Charter, Council of Europe, 6th May 1968
1.0 Introduction
Water, the subject matter of hydrology, is both commonplace and unique. It is found everywhere in the Earth’s ecosystem and it is essential to all known forms of life. The Earth is called the ‘Blue Planet’ because most of its surface (71%) is covered by water, although the total amount of water is under 0.5% of the Earth’s total volume. About 97% (depending on the method of calculation) occurs as saline water in the seas and oceans. Of the 3% that is fresh water considerably more than half is locked up in ice sheets and glaciers, and another substantial volume occurs as virtually immobile deep groundwater that is not easily accessible. The really mobile fresh water, which contributes frequently and actively to rainfall, evaporation and streamflow, represents only about 0.02% of the global total (see Figure 1.1).
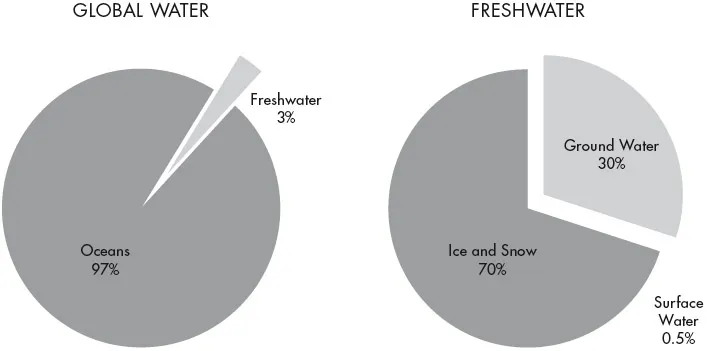
Fig 1.1: Global water constituents: Freshwater comprises only 3% of the total global water, and less than 0.5% of that freshwater is present as surface waters (Table 1.1).
A reliable source of water is the essential basis for human civilisation, and an integral part of the natural world. Without water every form of life on Earth would stop. Indeed, water makes up the bulk of most living things and it is a major medium for transporting energy, dissolved chemicals and sediments at scales from the molecular to the global. Moving water and ice are the agents of erosion and deposition. However, water creates risk to humans, in the form of floods and droughts, and polluted water harbours disease. Water resources for human exploitation must be allocated and used in a sustainable and equitable way to serve the competing and often conflicting requirements of agriculture, industry, households, power generation, navigation, flood protection and recreation, while maintaining a healthy environment. Taken for granted where plentiful, a prized possession where scarce, water is the only naturally occurring inorganic liquid, and is the only chemical compound that occurs in normal conditions as a solid, a liquid and a gas. The abundance of water is the feature that sets this planet apart from its fellows in the solar system.
Hydrology is defined formally as “the science which deals with the waters of the Earth, their occurrence, circulation and distribution on the planet, their physical and chemical properties and their interactions with the physical and biological environment, including their responses to human activity” (UNESCO, 1964). This book focuses on the principles and processes of hydrology which are concerned largely with physical or environmental hydrology. In this context, water is viewed in the same way as soil, vegetation, climate or rock, as an element of the landscape to be investigated and ultimately understood by means of rigorous, scientific examination and analysis. The science of hydrology overlaps with many other sciences. The study of precipitation and evaporation lie within the realm of the meteorologist, the storage of water in the soil and its percolation to groundwater are of direct relevance to agriculturalists, soil physicists and geologists. The flow of water in the river is the province of the hydraulic engineer. Only the hydrologist deals with these fragmented aspects of the hydrological cycle in its entirety (McCulloch, 1975). The aim of hydrology is to seek knowledge and understanding of the hydrological cycle (see Figure 1.3) in ways that lead to its safer exploitation, more reliable prediction and more effective control of water and water resources.
Hydrology is of fundamental importance in managing water, and seeks to understand the movement of water through the environment, and predict how water bodies will behave under different circumstances. At its broadest, hydrology encompasses all aspects of water as it moves through the water cycle, but is more usually taken to focus upon water on the land surface and in the soil profile, rather than in the air or the sea
Specifically, hydrology – and hydrologists help to provide a safer and better quality of life for people, and an enhanced environment for wildlife through:
•Securing water supplies for public use, including drinking water and sanitation,
•Ensuring the provision of water for food production, crops and livestock,
•Protecting against, and giving warning of, approaching floods and droughts to reduce their impact through economic and physical damage and loss,
•Protecting people and the environment from pollution and over-abstraction,
•Maintaining and improving aquatic habitats for wildlife, navigation, and recreation.
1.1 Water – facts and figures
Water is present on Earth in three phases. In its liquid form, precipitation meets the basic water needs of humans, animals, and plants. Its runoff into streams sustains ecosystems and, along with percolation into aquifers, ensures long term storage and supply for human uses. The oceans are the world’s primary source of water vapour that feeds precipitation. Atmospheric water vapour is a greenhouse gas, allowing much of the sun’s shortwave radiation to pass through but absorbing the longwave radiation emitted by the Earth’s surface, which results in the Earth’s surface temperature being about 30°C warmer than it would be otherwise (Trenberth, 1992). In addition, water vapour may condense into clouds that reflect and absorb solar radiation, thus directly affecting the Earth’s radiant energy balance. In water’s frozen form, sea ice and snow cover tend to cool the planet by reflecting the incoming solar radiation. Glaciers, especially those at mid-latitudes, provide water storage and summer supply for both agriculture and urban areas around the world.
As the world population increases towards 10 billion by 2050, and climate change progresses, increasing stresses will be placed on water resources. The global distribution of fresh water over the globe is amazingly uneven in both space and time, and many regions of the globe currently suffer from water scarcity. Even is the case of Britain, a generally humid land, the spatial pattern of rainfall and runoff (greater in the North and West) is largely the reverse of the distribution of its population (higher in the South and East), creating problems of water supply. In fact by the World Bank criterion (<1,000 m3 per head per year of available water) much of South East England can be classed as suffering from serious water stress.
Water is the only naturally occurring liquid most people see, and it is usually thought of as being a common, ordinary substance. But this colourless, tasteless, odourless fluid is far from being ordinary; water is one of the most extraordinary substances and defies many of the normal laws of physics and chemistry. It is its unusual attributes that make water of unique importance to life across the globe and to humanity.
1.1.1 The special characteristics of water
Water combines two of the most common elements, but it does not behave like any other substance. Hydrogen and oxygen atoms form a strong covalent bond with electrons shared between them. Due to the distribution of electrons the oxygen side of the water molecule has a negative charge and the side with the hydrogen atoms has a positive charge. This means that the positive end of one molecule will attract the negative end of another water molecule to form a hydrogen or polar bond (Figure 1.2). This weak electrostatic attraction between molecules has only about 1/10 of the strength of a covalent bond within a molecule, yet determines most of water’s unique properties:
Hydrogen bonds between water molecules result in water’s cohesiveness - literally “sticking” to itself, giving it a high surface tension. Hydrogen bonds also form between water and a solid such as a soil particle. This is especially important to the movement of water in soil, and plants make use of capillary rise to draw water up through their stems to their leaves.
•Most substances shrink as they cool, but when water falls below 4°C it starts to expand and become lighter. That is why a water body such as the lake freezes from th...
Table of contents
- Cover
- Half Title
- Dedication
- Title Page
- Copyright Page
- Contents
- Preface
- Chapter 1: Introduction
- Chapter 2: Precipitation
- Chapter 3: Interception
- Chapter 4: Evaporation
- Chapter 5: Groundwater
- Chapter 6: Soil Water
- Chapter 7: Streamflow
- Chapter 8: Water Quality
- Chapter 9: Hydrology in a changing world
- References
- Index
- Acknowledgements
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Hydrology by M. Robinson,R. C. Ward in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Environmental Science. We have over one million books available in our catalogue for you to explore.