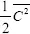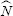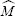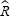![]()
CHAPTER 1
INTRODUCTION
1.1 BACKGROUND INFORMATION ON GASES
1.1.1 AIR COMPOSITION AND AIR MOLECULES
Air is often described in terms of its properties, including its density, temperature, and pressure. A typical density given for air is about 1.2 kg/m3, which is almost exact for 21°C at 1 atmosphere. Consequently, the mass of the air in 1 m3 of volume is about 1.2 kilograms. From chemistry, we know that one kilogram mole (kmol) of any substance has 6.02252 × 1026 molecules. The typical composition for dry air is given as 78.08 percent nitrogen (N2), 20.95 percent oxygen (O2), 0.93 percent argon (Ar), and about 0.04 percent carbon dioxide (CO2). If the percentages are multiplied by the individual molecular weights of these main four constituents, air is found to have a composite molecular weight of 28.97 kg/kmol. Consequently, 1 m3 of volume will typically contain nearly 2.5 × 1025 molecules of air, which is a nearly incomprehensible number. The root mean square of the speed of these molecules is around 503 m/s, and the average distance they travel before bumping into another molecule (the mean free path, λ) is about 66 nm. This means an average air molecule at 1 atmosphere and 21°C experiences about 7.6 billion collisions a second.
1.1.2 TEMPERATURE AND GASES
Air is largely made up of diatomic molecules, molecules with two atoms. These air molecules can store energy through their kinetic energy of motion, but also through rotational energies in two axes. The combination of kinetic energy through velocity or translation plus this energy of rotation is often called the internal energy of the gas. Generally, each of the
directions of translation and each axis of rotation can store equal amounts of energy. This equivalence between energies of the three directions of translation and the two independent directions of rotation is called the equipartition of energy. About 0.93 percent of air is Argon a monotonic gas. The internal energy of this monotonic gas molecule is related to one-half the mean squared speed of the molecules
times the mass,
m, of the given molecule. In fact, the mean energy of translation for all the molecules in the gas can be quantified in a similar manner.
The energy of translation can also be estimated based on its temperature.
Here,
T is the absolute temperature and
k is the Boltzman constant (
k = 1.38054×10
-23 J/molecule/K, note this term is only used in Chapter 1), which is essentially the gas constant per molecule. Consequently, absolute temperature is related directly to the mean squared speed of the molecules. This relationship can be put into more familiar terms by multiplying the right-hand side of both equations by Avogadro’s number,
. The new equality becomes:
Here,
is the (composite) molecular weight of the gas, and
is the universal gas constant (8314.46 J/kmol/K). The square root of the mean squared speed of the molecules can be determined using equation (3) yielding:
From this relationship, it is apparent that both the square root of the mean squared speed of a molecular species or a composite gas like air is related to the molecular weight. At 21°C (294.15 K), using the composite molecular weight of air (28.97 kg/kmol), the composite velocity is determined to be around 503 m/s. Nitrogen, which is slightly lighter, will have a slightly higher speed on average, while oxygen and the other molecules in air, which are heavier, will move at slightly lower average speeds.
Later, the speed of sound of air will be derived from a macroscopic approach. This analysis will show that the speed of sound of air is directly related to the absolute temperature of air similar to the square root of the mean squared speed of a molecule. Note that the square root of the mean squared speed of a molecule is slightly different than the average or mean speed of molecular motion. However, this speed in equation (1.4) can be determined easily from energy concepts related to molecular motion. In general, in thinking about molecular motion, realize that molecular speeds have a random distribution, which is described mathematically by the famous Maxwellian distribution. It turns out that the root mean square speed (equation 1.4) is slightly higher than the average speed, which is slightly higher than the most probable speed. At a given total temperature, the maximum bulk velocity that a gas can achieve from expansion can be determined from energy concepts. Basically, if all the energy stored in a gas is applied to its expansion to maximize the bulk velocity, for a given total temperature for a gas, the maximum velocity will be:
This equation means that all of the random and organized energy in the gas, including translational and rotational energies and flow work, is converted into kinetic energy. Here, the CP is the specific heat at constant pressure per mass for the composite gas (J/kg/K) and T is the absolute temperature (K).
1.1.3 PRESSURE AND GASES
The concept of pressure can be related to the force that a gas exerts on a surface in a volume. A simple thought experiment would be to think of a gas as consisting of a very large number of molecules. However, instead of considering the huge number of collisions, which occur within a gas in a volume, let us think of a gas molecule as a point in space that has mass and velocity, but that takes up such a small volume that collisions do not need to b...