
- 217 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
Inhalt des Buchs sind die Grundlagen der Elektrotechnik. Dabei richtet sich die inhaltliche Tiefe und die Darstellung an Studierende des Fachs Mechatronik, insbesondere an solche mit einem mechanischen oder maschinenbaulichen Hintergrund. Dementsprechend behandelt das Buch die Grundlagen der Elektrotechnik unter dem Blickwinkel dieser Vorbildung der Studierenden. Ziel ist es, diese Grundlagen durch Praxisbeispiele von einem mechatronischen Beispielsystem so zu veranschaulichen, dass der Einsatz der Theorie in der Praxis klar wird.
Als mechatronisches Beispielsystem wird ein Auto bzw. elektrische und mechatronische Systeme im Automobil verwendet, um den Studierenden einen direkten Praxisbezug der Grundlagen aufzuzeigen. Dazu werden passende Anwendungen im Anschluss an einzelne Abschnitte des Buchs dargestellt, die den direkten Bezug des Gelernten in der Praxis geben.
Um mechatronische Systeme, die z.B. für neue Produkte immer wichtiger werden, verstehen und entwickeln zu können, müssen fundierte und praxisbezogene Kenntnisse vorhanden sein. Dies betrifft insbesondere auch die Elektrotechnik.
Neben den theoretischen Teilen und den Praxisanwendungen gibt es zahlreiche Übungsaufgaben, um das Gelernte anzuwenden und zu vertiefen. In wie weit die Übungen in das Buch integriert werden ist noch zu klären. Ergänzend gibt es noch eine kurze Einführung in das Simulationsprogramm PSPICE, mit dem die Studierenden weitergehende Erkenntnisse erlangen können.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1 The fundamentals of solid-state physics
1.1 Charge carriers, crystal structure and conductivity
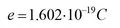
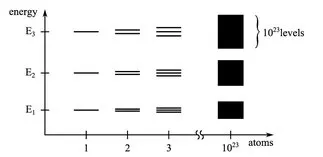
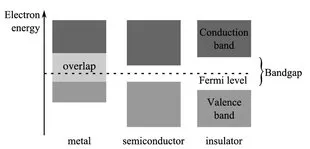
1.2 The electrical properties of solids
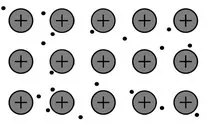
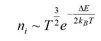
Table of contents
- The Fundamentals of Electrical Engineering
- Title Page
- Copyright Page
- Preface
- Table of Contents
- 1 The fundamentals of solid-state physics
- 2 Fundamental electrical laws
- 3 Fundamental circuit elements
- 4 Fundamental electrical circuit laws
- 5 Circuit analysis
- 6 Operational amplifier
- 7 Time domain circuit analysis
- 8 Building blocks
- 9 AC power
- 10 Oscillating circuits
- 11 Semiconductor devices
- 12 Circuit simulation
- References
- Index