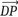1.1 Why polymers?
1.1.1 Generality
Polymers are everywhere in our everyday life. Both synthetic and natural polymers have unique properties (chemical, mechanical, electrical, thermal), which make them irreplaceable in the particular job they are called to do. The most impressive aspect in the world of polymers is the enormous variety of applications, which span from everyday consumer goods (house wares, toys, bottles, packaging, textiles, furniture), heavily used electrical and electronic items (computer, cable insulation, household appliances), construction (housing, pipes, adhesives, coatings, insulation), transportation (tires, appliance parts, hardware, carpeting), medical and hospital furniture and goods (gloves, syringes, catheters, bandage), to high-performance, sophisticated materials for aerospace, bulletproof vests, nonflammable fabric, artificial organs, degradable device for controlled release of drugs or chemicals, high-power-density batteries, high-strength cables for oceanic platforms, etc.
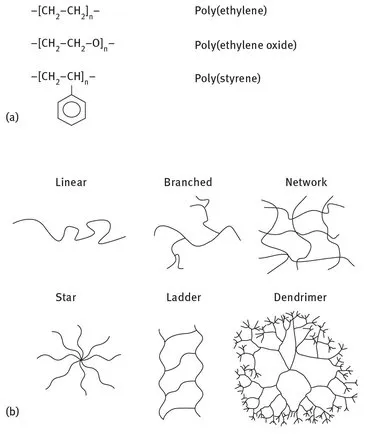
From a chemical point of view, polymers are high-molecular-weight compounds made up of simple repeating units called monomers. Molecular weights range from thousands to millions of atomic mass units, which makes writing a definitive molecular structure for these materials close to impossible. Polymer structures are therefore represented by enclosing the repeating unit (monomer) in brackets and placing “n” as subscript (Fig. 1.1a).
Polymers having more than one kind of repeating units are called copolymers; the units can be distributed either randomly in the chains or in an ordinate alternate sequence or in blocks. Moreover, the chains of the polymer structure may arrange in linear, branched, network, stars, ladder, or dendrimer fashion (Fig. 1.1b). Complex structures such as networks are formed when cross-linking occurs, i.e. the formation of covalent bonds between polymer chains; this process leads to a remarkable increase in molecular weight, and this in turn strongly influences chemical, physical, and mechanical properties of the material as well as its processability.
1.1.2 Synthesis
The first synthetic polymer that was produced on a commercial scale was Bakelite, a phenol-formaldehyde resin (by its inventor, the Belgian chemist Leo Baekeland [1]), at the beginning of 1900; however, it is the German chemist Hermann Staudinger who is rightly recognized as the father of the chemistry of macromolecules; for his studies on the relationship between structure of polymers and their properties, he received the Nobel Prize in Chemistry in 1953. During those years, polymer chemistry experienced the most significant advances and the beginning of a true revolution in the polymer industry, thanks to the work of Giulio Natta in Italy, who, for the first time, synthesized polymers having controlled stereochemistry [2] using the coordination catalysts developed by the German chemist Karl Ziegler [3]. The two scientists were awarded by the Nobel Prize in Chemistry in 1963. It was clearly demonstrated that polymers with similar chemical composition but exhibiting different molecular orientation have different morphology and their mechanical properties too may differ markedly. It is therefore vital, in view of commercial exploitation of polymer materials, to have full knowledge and control of the polymerization process. In brief, two reaction mechanisms are possible: step reaction (generally, a condensation reaction) and chain reaction (generally, an addition reaction). In the former, poly-functional monomers react randomly to give dimers, trimers, and oligomers, which can react with other monomers or among themselves: monomers are consumed rapidly, but polymer chains do not grow so much (i.e. the increase in molecular weight is slow). Polyamides (e.g. nylon 66) and polyesters (e.g. polyurethane) are produced by this reaction mechanism. In the chain reaction, however, an initiator (generally a free radical) starts the reaction by opening the double bond of a vinyl monomer and adding to it, with one electron remaining unpaired. The reaction propagates through the successive linking of a monomer to the end of the growing chain; finally, two free radicals react to end each other’s growth activity (termination). Monomers are consumed more slowly with respect to step reactions, but there is a considerable quick increase of the chain molecular weight. Polytetrafluoroethylene (Teflon), polyethylene, and polystyrene (PS) are important examples of polymers obtained by chain reaction mechanism.
Fig. 1.1: (a) Conventional representation of polymers structures. (b) Arrangement of chains in the polymer structure.
In polymer synthesis, it can be assumed that each chain reacts independently. Therefore, the
bulk polymer is characterized by a wide distribution of molecular weights and chain lengths, and these factors primarily determine the properties of polymers. The degree of
polymerization (DP) is the number of repeat units (included chain ends) in the chain and gives a measure of molecular weight; due to the presence of chains of varying lengths in any polymer, the average DP,
, is used more. Moreover, to characterize the distribution of polymer lengths in a sample, two parameters are defined: number average (
Mn) and weight average (
Mw) molecular weight. The number average is the sum of individual molecular weights divided by the number of polymers. The weight average is proportional to the square of the molecular weight. Therefore, the weight average is always larger than the number average (unless all molecules possess the same weight; then
Mn =
Mw).
1.1.3 Classification and nomenclature
Polymers are classified in many ways, the most common classifications being (1) based on source (natural, semisynthetic, or synthetic); (2) based on structure (linear, branched chain, cross-linked, or network); (3) based on molecular forces, which determine the mechanical properties (elastomers, fibers, thermoplastic polymers, or thermosetting polymers); (4) based on polymerization mode (addition and condensation polymers, which are currently referred to as chain growth polymers and step growth polymers, respectively).
The reported classifications, although useful from a practical point of view, are, in some cases, a rough simplification. For example, it must be emphasized that not all step reactions are condensation processes and not all the chain reactions are addition processes; this explains why IUPAC classified the polymerization processes [4] in a more comprehensive manner with respect to the above-simplified scheme.
At the same time, an IUPAC commission elaborated a series of rules for polymer nomenclature [5]. This is quite a complicated matter: some details and significant examples have been simply and clearly reviewed in many textbooks on polymers (see e.g. [6-7-8-9]), but this is outside the scope of this book. However, readers should be familiar with the most widely accepted terminology, and therefore, some general information is provided. Polymers (and copolymers) are grouped in families according to the functional group(s) present in the repeating units when their structure is easily identifiable: polyesters, polyethers, polyimides, polyamides, polyamideimides, etc. In the presence of structures that cannot be easily defined, (co)polymer nomenclature is based on the name of the starting monomer(s). This approach is referred to as source-based system and is more widely used than the structure-based IUPAC nomenclature. Moreover, when the starting monomers contain a carbon-carbon double bond, the resulting polymers are classified as vinyl polymers, in all the other cases, they are referred to as nonvinyl polymers: the nomenclature of latter may be quite complex, and the source-based approach once again is more generally used than IUPAC rules. Depending on the approach used, terminology may be quite different; to give an example, (-CH2-CH2-)n is named polyethylene or poly(methylene) in the source-based and IUPAC approaches, respectively, and similarly, (-CH2CHCOOH-)n is named poly(acrylic acid) or poly(1-carboxylatoethylene), respectively. Given the complexity of polymer nomenclature, its abbreviations and/or trade terms have been and still are widely used both in the scientific literature and in the industrial world. Many of these terms are so popular and widely recognized that they are commonly used in the everyday language as well (PVC, PE, PET,...