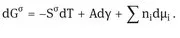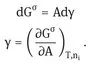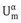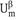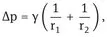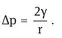1.1 Definition of the interfacial region and interfacial tension
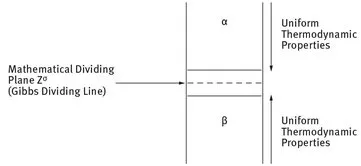
When two immiscible phases α and β come into contact, an interfacial region develops. The interfacial region is not a layer that is one molecule thick; it is a region with thickness δ with properties different from the two bulk phases α and β. In bringing phases α and β into contact, the interfacial regions of these phases undergo some changes, resulting in a concomitant change in the internal energy. If we were to move a probe from the interior of α to that of β, one would at some distance from the interface start to observe deviations in composition, in density, in structure; the closer to phase β the larger the deviations until eventually the probe arrives in the homogeneous phase β. The thickness of the transition layer will depend on the nature of the interfaces and on other factors. For solid surfaces and solid-liquid interfaces it is usually not more than a few atomic layers thick, but in the presence of diffuse double layers it can become tens or even hundreds of nanometers thick. Gibbs [1] considered the two phases α and β to have uniform thermodynamic properties up to the interfacial region. He assumed a mathematical plane Zσ in the interfacial region in order to define the surface or interfacial tension γ. A schematic representation of the interfacial region and the Gibbs mathematical plane is given in Fig. 1.1.
Fig. 1.1: The Gibbs dividing line.
Using Gibbs’ model, it is possible to obtain a definition of the surface or interfacial tension γ. The surface free energy dGσ is made of three components: an entropy term, SσdT; an interfacial energy term, Adγ; a composition term Σnidμi (ni is the number of moles of component i with chemical potential μi).
The Gibbs–Deuhem equation is,
At constant temperature and composition,
For a stable interface γ is positive, i.e. if the interfacial area increases, Gσ increases. Note that γ is energy per unit area (mJm−2) which is dimensionally equivalent to force per unit length (mNm−1), the unit usually used to define surface or interfacial tension.
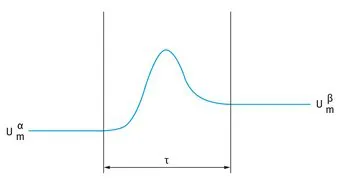
An alternative approach to Gibbs treatment was given by Guggenheim [2] in which two dividing planes are drawn, one in phase α, the other in phase β, as is illustrated in Fig. 1.2.
Fig. 1.2: Guggenheim convention of the interfacial region.
The planes are sufficiently far outside the interfacial region that beyond them the two phases have their bulk properties with internal energy
and
. In this convention the interfacial region is enclosed between two planes, a distance τ apart. The interfacial region has now a finite volume Aτ. In this convention the value of excess quantities depends on the choice of the two planes and hence on τ, whereas in the Gibbs convention it depends on the choice of one location of the dividing plane. The Guggenheim analysis [2] for the interfacial region is quite complex and hence the more simple Gibbs convention [1] is used in the analysis of interfacial phenomena.
For a curved interface, one should consider the effect of the radius of curvature. Fortunately, γ for a curved interface is estimated to be very close to that of a planer surface, unless the droplets are very small (< 10 nm).
Curved interfaces produce some other important physical phenomena which affect emulsion properties, e.g. the Laplace pressure Δp which is determined by the radii of curvature of the droplets,
where r1 and r2 are the two principal radii of curvature.
For a perfectly spherical droplet r1 = r2 = r and
For a hydrocarbon droplet with radius 100 nm, and γ = 50 mNm−1, Δp ∼ 106 Pa (∼ 10 atm).
1.2 Role of interfacial phenomena
In all disperse systems such as suspensions, emulsions, foams, etc., the structure of the interfacial region determines its colloidal properties [3–6]. The larger the interfacial area, i.e. the larger the surface to volume ratio of the particle or droplet, the more important the role of the structure of the interfacial region. The colloid stability /instability of any disperse system is determined by the property of the interfacial region. In actual fact colloid and interface science are one individual subject. This is particularly the case with charged interfaces that form electrical double layers and those interfaces that contain adsorbed surfactants and/or polymers. With systems containing electrical double layers, repulsion between the particles or droplets takes place as a result of the overlap of double layers. This is particularly the case at low electrolyte concentrations and low valency of the indifferent electrolyte. This double layer repulsion overcomes the van der Waals attraction and at intermediate distances an energy barrier is produced that prevents approach of the particles. This barrier can reach several kT units (where k is the Boltzmann constant and T is the absolute temperature) which becomes much higher than the thermal motion (∼ kT) and this prevents particle aggregation (flocculation or coagulation). As the electrolyte concentration is increased, the range and magnitude of the repulsive energy is reduced and at a critical concentration (defined as the critical coagulation concentration, ccc) fast flocculation and irreversible aggregation occur. With adsorbed nonionic surfactants or polymers an adsorbed layer with thickness δ is produced. When the particles or droplets approach a surface-surface distance h < 2δ, strong repulsion occurs due to the unfavorable mixing of the adsorbed chains when these are in good solvent conditions. This repulsion is referred to as steric interaction and at distances < 2δ a very sharp increase in repulsion energy occurs when h < 2δ. This steric repulsion overcomes the van der Waals attraction at h ∼ 2δ. The repulsion produced by the presence of adsorbed layers of surfactant or polymers is generally more effective than the electrostatic repulsion produced by overlap of the double layers. The stability is less sensitive to addition of moderate electrolyte concentration, provided the medium remains a good solvent for the chains.
The field of colloid and interface science has no boundary since chemists, physicists, engineers, biologists, mathematicians can all be engaged in the field. For successful applications in industry, multidisciplinary teams are required. Understanding the basic principles of colloid and interface Science will enable industry to develop many complex systems in a shorter period of time. Most colloidal systems used in industry are multiphase and complex formulations. They may contain more than one disperse phase, e.g. suspension/emulsion systems (suspoemulsions).
For convenience, I will list the topics of colloid and interface science under two main headings: disperse systems and interfacial phenomena. This subdivision does not imply any separation for the following reasons: All disperse systems involve an interface and many interfacial phenomena are precursors for formation of disperse systems, e.g. nucleation and growth, emulsification, etc. The main objectives of the present book are to cover the following topics: The basic principles that are involved in interfacial phenomena as well as the formation of colloidal dispersions and their stabilization; surfactants and polymer adsorption at various interfaces and interfacial phenomena in wetting, spreading and adhesion.
Several interfacial phenomena may be considered when dealing with colloidal dispersions: (i) Charge separation and formation of electrical double layers. (ii) Wetting of powders and the role of surfactants. (iii) Adsorption of surfactants and polymers at the solid/liquid and liquid/liquid interfaces.
Several examples of disperse systems can be quoted to illustrate the role of interfacial phenomena. The first example is that of solid/liquid dispersions (suspensions) that find application in almost every industrial preparation, e.g. paints, dyestuffs, paper coatings, printing inks, agrochemicals, pharmaceuticals, cosmetics, food products, detergents, ceramics, etc. The powder particles can be hydrophobic, e.g. organic pigments, agrochemicals, ceramics or hydrophilic, e.g. silica, titania, clays. The liquid can be aqueous or nonaqueous. It is essential to understand the process of dispersion of powders in liquids (to prepare suspensions) at a fundamental level: dispersion is a process whereby aggregates and agglomerates of powders are dispersed into “individual” units, usually followed by a wet milling process (to subdivide the particles into smaller units) and stabilization of the resulting dispersion against aggregation and sedimentation [4]. There are two main processes for the preparation of solid/liquid dispersions. The first depends on the “build-up” of particles from molecular units, i.e. the so-called condensation method, which involves two main processes, namely nucleation and growth. In this case, it is necessary first to prepare a molecular (ionic, atomic or molecular) distribution of the insoluble substances; then by changing the conditions precipitation is caused leading to the formation of nuclei that grow to the particles in question. In the second procedure, usually referred to as a dispersion process, larger “lumps” of the insoluble substances are subdivided by mechanical or other means into smaller units. The role of interfacial phenomena in both processes is very clear. For example, in the process of nucleation and growth the interfacial region determines the size distribution of the particles formed. In the dispersion method, control of the interfacial region determines the process of wetting of the powder and its dispersion in single units. In addition, the interfacial region determines the wet milling process. Both effects are determined by the presence of surfactants (wetting and dispersing agents) which will reduce the solid/liquid interfacial tension thus facilitating the two processes. These adsorbed surfactants and/or dispersing agents also provide an effective repulsive barrier to prevent any flocculation of the final dispersion. This repulsive barrier can be produced with charged interfaces and formation of electrical double layers as mentioned above. The latter can be produced by dissociation of ionogenic groups or by adsorption of ionic surfactants. In many systems nonionic and polymeric surfactants are used as dispersant to provide effective steric stabilization [4]. A third case is that where the repulsion is produced by a combination of electrostatic and steric repulsion. Referred to as electrosteric stabilization, e.g. when using mixtures of ionic and nonionic surfactants or using polyelectrolytes as dispersants.
The second example is that of liquid/liquid dispersions or emulsions. The latter are a class of disperse systems consisting of two immiscible liquids [5]. The liquid droplets (the disperse phase) are dispersed in a liquid medium (the continuous phase). Several classes may be distinguished: Oil-in-Water (O/W); Water-in-Oil (W/O); Oil-in-Oil (O/O). The latter class may be exemplified by an emulsion consisting of a polar oil (e.g. propylene glycol) dispersed in a nonpolar oil (paraffinic oil) and vice versa. Several industrial systems consist of emulsions of which the following are worth mentioning: food emulsion, e.g. mayonnaise, salad creams, deserts, beverages, etc.; personal care and cosmetics, e.g. hand creams, lotions, hair sprays, sunscreens, etc.; agrochemicals, e.g. self emulsifiable oils which produce emulsions on dilution with water, emulsion concentrates (EWs) and crop oil sprays; pharmaceuticals, e.g. anesthetics of O/W emulsions, lipid emulsions, double and multiple emulsions, etc.; paints, e.g. emulsions of alkyd resins, latex emulsions, etc.; dry cleaning formulations which may contain water droplets emulsified in the dry cleaning oil which is necessary to remove soils and clays; bitumen emulsions which are emulsions prepared stable in containers but when applied to road chippings they must coalesce to form a uniform film of bitumen; emulsions in the oil industry – many crude oils contain water droplets (for example the North Sea oil) and these must be removed by coalescence followed by separation.
To disperse two immiscible liquids one needs a third component, namely the emulsifier. The choice of the emulsifier that adsorbs at the liquid/liquid interface is crucial in the formation of the emulsion and its long-term stability. The interfacial region determines the stability/instability of the emulsion. For example, flocculation occurs when there is not sufficient repulsion to keep the droplets apart to distances where the van der Waals attraction is weak. Flocculation may be “strong” or “weak”, depending on the magnitude of the attractive energy involved. Clearly the interfacial region must provide a sufficient repulsive barrier (either by double layer repulsion and/or steric repulsion) to prevent droplet aggregation. Another instability process is that of Ostwald ripening (disproportionation) which results from the finite solubility of the liquid phases. Liquids which are referred to as being immiscible often have mutual solubilities which are not negligible. With emulsions that are usually polydisperse, the smaller droplets will have larger solubility when compared with the larger ones (due to curvature effects). With time, the smaller droplets disappear and their molecules diffuse to the bulk and become deposited on the larger droplets. With time the droplet size distribution shifts to larger values. Ostwald ripening can be significantly reduced by proper control of the interfacial region. By using a polymeric surfactant that strongly adsorbs at the oil/water interface and does not desorb on droplet approach the Ostwald ripening rate can be significantly reduced. A third instability problem with emulsions is coalescence, which refers to the process of thinning and disruption of the liquid film between the droplets with the result of fusion of two or more droplets into larger ones. The limiting case for coalescence is the complete separation of the emulsion into two distinct liquid phases. The driving force for coalescence is the surface or film fluctuations which result in the close approach of the droplets whereby the van der Waals forces is strong thus preventing their separation. The role of interfacial phenomena in the liquid film separating the droplets is crucial in preventing coalescence. The magnitude of the electrostatic and steric repulsion in the liquid film must exceed the van der Waals attraction to prevent coalescence [5].
The third example where interfacial phenomena play a vital role is that of foam stability/instability. Foam is a disperse system, consisting of gas bubbles separated by liquid layers. Because of the significant density difference between the gas bubbles and the medium, the system quickly separates into two layers with the gas bubbles rising to the top, which may undergo deformation to form polyhedral structures. Pure liquids cannot foam unless a surface active material is present. When a gas bubble is introduced below the surface of a liquid, it bursts almost immediately as soon as the liquid has drained away. With dilute surfactant solutions, as the liquid/air interface expands and the equilibrium at the surface is disturbed, a restoring force is set up which tries to establish the equilibrium. Th...