
eBook - ePub
Grignard Reagents and Transition Metal Catalysts
Formation of C-C Bonds by Cross-Coupling
- 295 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Grignard Reagents and Transition Metal Catalysts
Formation of C-C Bonds by Cross-Coupling
About this book
In 1912, the Chemistry Nobel Prize was awarded for the discovery of the so-called Grignard reagents. Nowadays, many transition metal variants are developed to modify reactivity and selectivity of the C–C bond formation reaction.
The Grignard reaction is one of the fundamental organometallic reactions, often used in alcohol syntheses. With transition metals like iron, cobalt and nickel or with noble metals like copper, silver and palladium, modern Grignard reagents can be designed in reactivity, selectivity and functional group tolerance. This book, written by international experts, presents an overview on timely Grignard chemistry involving transition metals.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Grignard Reagents and Transition Metal Catalysts by Janine Cossy in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Analytic Chemistry. We have over one million books available in our catalogue for you to explore.
Information
David J. Nelson, Catherine S. J. Cazin, Steven P. Nolan
1Grignard Reagents and Palladium
1.1Introduction
One contributor to the success of palladium-catalyzed cross-coupling as a technique in academia and industry has been the ability to make use of a wide range of reagents that are commercially available and/or relatively straightforward to prepare. Grignard reagents [1]are some of the most common and widely known organometallic reagents in chemistry [2]. While these are relatively reactive species, they do not typically require the cryogenic conditions that their organolithium counterparts frequently demand. Yet, many coupling reactions of Grignard reactions occur at, or close to, room temperature.
The use of these valuable reagents in cross-coupling has been known for decades, since the studies of Kharasch et al. in the early 1940s [3, 4]. The cross-coupling of Grignard reagents with aryl halides is often referred to as the “Kumada-Tamao-Corriu reaction” (or simply, the “Kumada coupling”), but we have chosen not to ascribe a name to the reaction here.
In this chapter, we briefly examine the historical development of the cross-coupling of Grignard reagents, and discuss the modern applications of the palladium-catalyzed variants. While we focus here only on palladium catalysis, Shinokubo and Oshima have published a micro review that includes examples of catalysis with a wider range of metals [5], and Tamao has reviewed the development of the nickel-catalyzed variant [6]. We consider the reactions of different classes of Grignard reagent (aryl, vinyl, alkyl and alkenyl) with different classes of electrophile (aryl, vinyl, alkyl and alkenyl), with the aim of providing the chemist with an appreciation of what is possible using this method. We also briefly describe and discuss some examples from target syntheses, and from larger-scale industrial applications.
1.2The Discovery and Development of Catalytic Cross-Coupling Reactions of Grignard Reagents
The earliest studies originated from the laboratory of Kharasch, who demonstrated that various metal salts catalyzed the coupling of some aryl halides (and one alkyl halide) with phenyl magnesium bromide (Fig. 1.1) [3]; CoCl2 was shown to be the most efficacious (at loadings of 2.5–9 mol %), although MnCl2, FeCl2 and NiCl2 also showed some (lesser) catalytic activity.
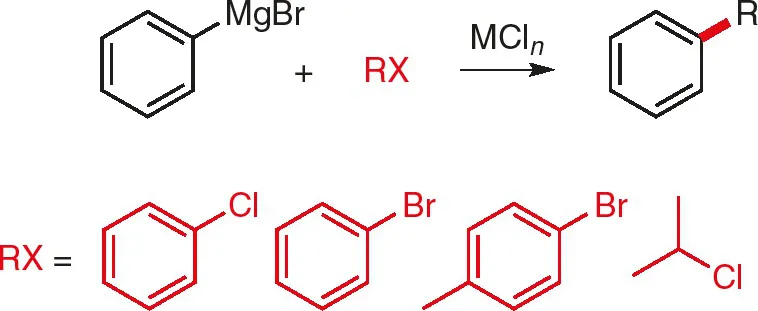
Fig. 1.1: Cross-coupling of phenyl magnesium bromide; MCln = CoCl2, MnCl2, FeCl2, or NiCl2 [3].
A subsequent study further probed the cobalt-catalyzed protocol, achieving the cross-coupling of phenyl-, cyclohexyl-, benzyl-, butyl- and naphthyl-magnesium bromide with several vinyl bromide and chloride compounds in the presence of typically 5 mol % CoCl2 [4]. However, the corresponding styrenes were not the only product, and in many cases were not even the major product; polymers and protodehalogenated products were also obtained. Therefore, while these studies represented a significant step towards catalytic coupling of Grignard reagents, these protocols were not practically useful for synthetic processes. Alternative systems based on iron(II) and iron(III) chloride complexes were disclosed by Kochi, in which alkylmagnesium bromides were coupled with vinyl bromides [7].
A watershed moment in the development of Grignard cross-coupling was the use of catalytic quantities of nickel complexes, at loadings as low as 0.1 mol % in some cases, to selectively prepare the desired products. It was at this point that the technique began to emerge as a potentially g...
Table of contents
- Cover
- Title Page
- Copyright
- Contents
- Contributing Authors
- Introduction
- Grignard Reagents and Palladium
- Grignard Reagents and Nickel
- Grignard Reagents and Iron
- Grignard Reagents and Cobalt
- Grignard reagents and Manganese
- Grignard reagents and Copper
- Grignard Reagents and Silver
- Index