
- 302 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Basic Principles of Interface Science and Colloid Stability
About this book
Volume 1 of the Handbook of Colloid and Interface Science is a survey of the theory of colloids in a variety of fields, as well as theircharacterization by rheology. It is an ideal reference work for research scientists, universities, and industry practitioners looking for a complete understanding of how colloids and interfaces behave.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1Introduction
1.1Colloidal dispersions
The word “colloid” was first coined by Thomas Graham [1] in 1861 who borrowed the word from the Greek word “kolla”meaning glue-like. Colloidal particles possess characteristic properties between those of true solutions (with molecules that can diffuse through membranes) and suspensions that can sometimes be easily observed by the naked eye. Clearly colloidal particles are unable to diffuse through membranes and when dispersed in a liquid medium they form a heterogeneous (two-phase) system that can scatter light. The best definition of colloids is perhaps “systems in which a significant proportion of the molecules lie in or are associated with interfacial regions”. Simple considerations suggest that the lowest limit for colloids (whereby one can distinguish between molecules in the interfacial region and the bulk) is 1 nm. The upper limit for colloidal dispersions lies in the region of 1000 nm (1 μm), whereby a significant proportion of the total molecules lie at the interface. Unfortunately, the exact range of colloid size is difficult to ascertain in an exact manner. For that reason Ostwald [2] described colloids as “the world of neglected dimensions”.
Colloidal particles are too small to be observed with ordinary optical microscopy. However, it was possible to demonstrate their existence using the technique of ultramicroscopic illumination first employed by Zsigmondy [3] in 1903. This is due to the fact that colloidal particles scatter light. The technique, known as dark field microscopy, works provided the refractive index of the particles is very much different from that of the medium so that sufficient light is scattered from them to be seen [4]. A schematic representation of the slit ultramicroscopic is shown in Fig. 1.1.
To visualize colloidal particles both scanning electron microscopy (SEM) and transmission electron microscopy (TEM) are effectively used with a resolution down to 5 nm for TEM. For metallic colloids (such as gold sols), a drop of the dispersion is deposited on a TEM grid, dried and observed directly in the microscope. The images, which are a two-dimensional representation, are captured on film or digitally and from these the size distribution is determined using an image analyser. For colloids such as organic polymers, proteins and biocolloids that can be damaged by the electron beam, a carbon or gold replica is prepared that is floated off the sample and observed in the microscope instead of the colloid. Many other techniques can be used for measuring the size of colloidal particles, of which light scattering is perhaps the most convenient to use. Both static (elastic) and dynamic (quasi-elastic) light scattering methods can be applied and these will be described in detail in Chapter 12, Vol. 2.

It should be mentioned that colloidal particles are not always spherical and many other shapes are encountered in practice, e.g. ellipsoids, rods, discs, etc. Measurement of particle size and shape distribution is essential since these determine many of the properties of the colloidal dispersion, such as its flow characteristics (rheology), solubility rate, stability, appearance of the dispersion , processing, etc. The particles are described as monodisperse if they are of the same size, otherwise they are polydisperse. It is therefore important to determine the particle size distribution and polydispersity index as will be discussed in detail in Chapter 12, Vol. 2.
As mentioned above, a colloidal dispersion is a two-phase system in which one phase (the disperse phase) is dispersed in a second continuous phase (the dispersion medium). The disperse phase can be solid, liquid or gas and the same applies for the dispersion medium. On this basis one can distinguish several classes of colloidal dispersions as shown in Tab. 1.1.
Tab. 1.1: Classes of colloidal dispersions.
| Solid | Liquid | Suspension |
| Liquid | Liquid | Emulsion |
| Gas | Liquid | Foam |
| Liquid | Gas | Aerosol |
| Liquid | Solid | Gel |
| Solid | Gas | Smoke |
| Solid | Solid | Composite |
Several examples of dispersion classes can be quoted. One of the earliest colloidal dispersions of the solid/liquid type is colloidal gold which was used in the fourth and fifth century BC by ancient Egyptians and Chinese to make ruby glass and for colouring ceramics [4]. Michael Faraday [6] prepared the first pure colloidal gold dispersion by reducing a solution of gold chloride with phosphorous. He recognized the colour of the dispersion was due to the small size of the gold particles. Nowadays such small sized particles covering the size range 1–100nm are referred to as nanoparticles. The early work of Brown on the random drifting of dispersed particles induced by thermal energy (referred to as Brownian motion) was given a theoretical treatment by Einstein [7]. Brownian motion of colloidal particles and the resultant dynamics are unique and important characteristics of colloidal systems [4].
A good example of naturally occurring colloid of the liquid/liquid type is milk, which consists of fat droplets dispersed in aqueous medium that also contains casein micelles which are also colloidal in nature. When milk is first obtained from cows, the fat droplets may exceed 1 μm and this results in creaming of these droplets. However, when milk is homogenized (using high pressure homogenizers) the fat droplets are subdivided into submicron droplets (nanodroplets) and this prevents the process of creaming.
An example of gas/liquid system (foam) is the beer head. When beer is poured into a glass one observes a head of foam and the air bubbles are stabilized by protein present in the beer. Several examples of liquid/gas dispersions (aerosols) can be quoted, such as fog or mist. One of the main applications of aerosols is in pharmacy for oral and topical use. Pharmaceutical aerosols are dosage forms containing therapeutically active ingredients intended for topical administration, introduction into body cavities, or by inhalation via the respiratory tract. The aerosol product consists of two components, namely concentrate containing the active ingredient and propellant(s). The latter provides the internal pressure that forces the product out of the container when the valve is opened and delivers the product in the desired form.
Liquid/solid dispersions or gels are semisolids consisting of a “three-dimensional” network in which the liquid is entrapped. The network can be either suspensions of small particles or large organic molecules (polymers) interpenetrated with liquid. In the first case, the inorganic particles, such as bentonite, form a three-dimensional “house of cards” structure through the gel. This is a true two-phase system. With polymers, either natural or synthetic, the molecules tend to entangle with each other due to their random motion. These systems are actually single phase in the macro-sense; the organic molecules are dissolved in the continuous phase. However, the unique behaviour of polymers, leading to high viscosities and gel formation, makes it possible to consider the gel as a two-phase system on the micro-level; the colloidal polymer molecule and the solvent. Gels find use as delivery systems for oral administration, for topical drugs applied directly to the skin or eye as well as for long acting forms of drugs.
Examples of solid/gas dispersions are smoke and dust as well as particles produced in coal fires and diesel engines. Several examples of solid/solid dispersions can be quoted such as painted glass, pigmented plastics as well as dispersions of silica in plastic to enhance the mechanical properties of the system.
1.2Self-assembly systems
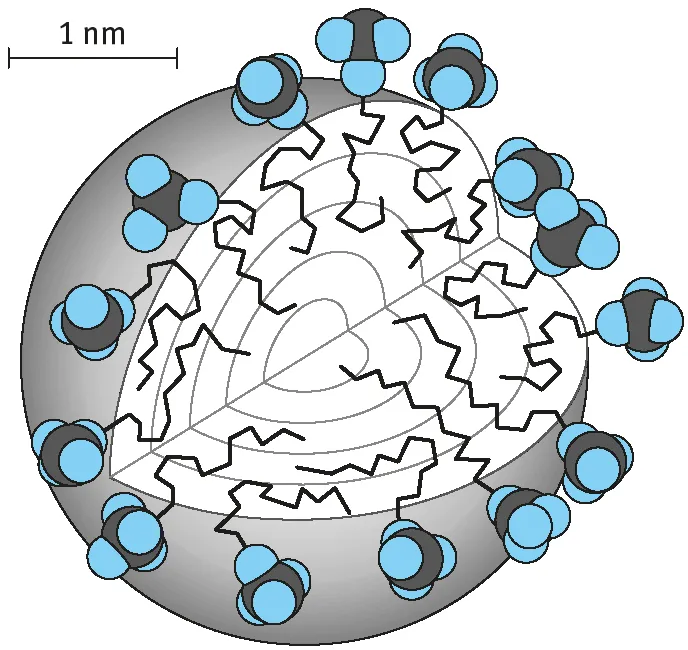
Certain organic molecules such as surfactants (generally described as amphiphiles) tend to associate in solution above a critical concentration to form aggregate structures (referred to as micelles) with dimensions in the colloid range. A good example is the association of sodium dodecyl sulphate (SDS) at concentrations ≥ 8.1 × 10−3 mol to form spherical micelles [8] as represented in Fig. 1.2. However, the micelles can be of different shapes (rod-shaped or lamellar) but still with one dimension in the colloid range. The different shapes of micelles are illustrated in Fig. 1.3. The size and shape of any micelle is determined by the surfactant structure and the environment. The surfactant structure determines the critical packing parameter of the molecule (the ratio of the cross-sectional area of the hydrocarbon chain to that of the head group). The head group cross-sectional area is determined by the size of that group which in turn is determined by electrolyte addition. For example, a spherical micelle may change to a rod-shaped one on addition of electrolyte.

1.3Interfacial phenomena
In all disperse systems such as suspensions, emulsions, foams, etc., the structure of the interfacial region determines its colloidal properties [9–13]. The larger the interfacial area, i.e. the larger the surface to volume ratio of the particle or droplet, the more important the role of the structure of the interfacial region. For convenience, I will list the topics of colloid and interface science under two main headings, namely disperse systems and interfacial phenomena. This subdivision does not imply any separation for the following reasons: All disperse systems involve an interface and many interfacial phenomena are precursors for formation of disperse systems, e.g. nucleation and growth, emulsification, etc. The main objective of the present book is to cover the following topics: The basic principles that are involved in interfacial phenomena as well as the formation of colloidal dispersions and their stabilization, as well as surfactants and polymer adsorption at various interfaces and interfacial phenomena in wetting, spreading and adhesion.
Several interfacial phenomena may be considered when dealing with colloidal dispersions:
(i)Charge separation and formation of electrical double layers.
(ii)Wetting of powders and the role of surfactants.
(iii)Adsorption of surfactants and polymers at the solid/liquid and liquid/liquid interfaces and the role of the structure of the interfacial region.
The colloid stability/instability of any disperse system is determined by the property of the interfacial region. In actual fact, colloid and interface science are one individual subject. This is particularly the case with charged interfaces that form electrical double layers and those interfaces that contain adsorbed surfactants and/or polymers. With systems containing electrical double layers, repulsion between the particles or droplets takes place as a result of the overlap of double layers. This is particularly the case at low electrolyte concentrations and low valency of the indifferent electrolyte. This double layer repulsion overcomes the van der Waals attraction and at intermediate distances an energy barrier is produced that prevents approach of the particles. This barrier can reach several kT units (where k is the Boltzmann constant and T is the absolute temperature) which becomes much h...
Table of contents
- Cover
- Title Page
- Copyright
- Preface
- Contents
- 1 Introduction
- 2 Origin of charge at interfaces and structure of the electrical double layer
- 3 Electrokinetic phenomena and zeta potential
- 4 Double layer repulsion
- 5 Van der Waals attraction
- 6 Theory of colloid stability
- 7 Flocculation of colloidal dispersions
- 8 Association colloids
- 9 Adsorption of surfactants at the liquid/liquid interface
- 10 Adsorption of surfactants at the solid/liquid interface
- 11 Polymers and polymeric surfactants and their association
- 12 Adsorption and conformation of polymeric surfactants at interfaces
- 13 Steric stabilization
- 14 Flocculation of sterically stabilized dispersions
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Basic Principles of Interface Science and Colloid Stability by Tharwat F. Tadros in PDF and/or ePUB format, as well as other popular books in Ciencias físicas & Química industrial y técnica. We have over one million books available in our catalogue for you to explore.