![]()
Chapter 2
Rudolph KL (ed): Molecular Mechanisms of Adult Stem Cell Aging.
Else Kröner-Fresenius Symp. Basel, Karger, 2010, vol 1, pp 17–37
______________________
Hematopoietic Stem Cell Aging and Fate Decision
Anett Illinga · Zhenyu Jub
aInstitute of Molecular Medicine and Max Planck Research Department on Stem Cell Aging, University of Ulm, Ulm, Germany; bStem Cell Biology and Aging Research, Sino-German Laboratory for Aging and Regenerative Medicine, Institute of Laboratory Animal Sciences, Chinese Academy of Medical Sciences, Beijing, PR China
______________________
Abstract
The following section will give an overview of the current data and theories on the aging of hematopoietic stem cells (HSCs). Aging is characterized by a gradual loss of regenerative capacity to sufficiently maintain tissue homeostasis and organ integrity. Mounting evidence suggests that a decline in functional stem cells can contribute to diminished homeostatic maintenance and regenerative potential in tissues during aging. Long-lived stem cells are prone to accumulate precancerous damage. Anti-tumor mechanisms restrain potentially malignant stem cell clones by inducing apoptosis or senescence, but simultaneously provoke aging. Clarification of molecular changes in aging HSCs will not only help our understanding of age-related bone marrow failure and immuno-senescence, but will also provide insights into the molecular basis for increased lympho-hematopoietic malignancies during aging. An understanding of these age-dependent processes could ultimately provide a rational basis for the development of new treatment and prevention strategies for age-associated bone marrow failure, immune dysfunction and leukemia development.
Copyright © 2010 S. Karger AG, Basel
Characteristics of Hematopoietic Stem Cells
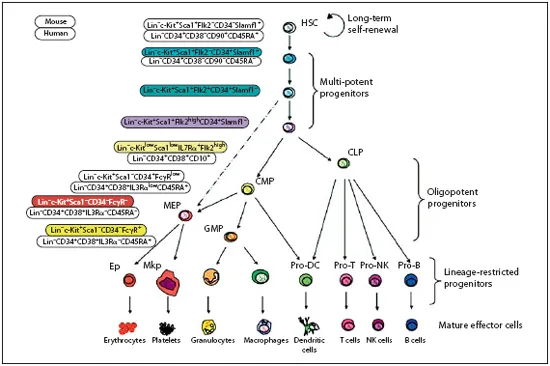
Hematopoietic stem cells (HSCs) are the best-characterized adult or somatic stem cells in the mammalian system [1]. HSCs ensure the maintenance and the development of all blood components, providing about 1011—1012 new blood cells daily in humans [2]. A hierarchical structure in the hematopoietic system has been well documented. The most primitive stem cells reside at the apex of the hierarchy and are capable of both self-renewal and generation of progenitor cells that differentiate into a variety of mature cell types (fig. 1) [3, 4].
The most primitive HSCs are named long-term HSCs (LT-HSCs), which can be identified via a broad range of surface markers [5–11]. LT-HSCs are negative for lineage markers, negative for CD48, CD244, CD34, Flt3, and N-cadherin, but positive for Tie2, endoglin, Thy1 (preferably expressed at lower levels), Sca1, cKit, CD38 and CD150. Additionally, LT-HSCs, like several tumor cells, have been shown to express the P-gly-coprotein, a member of the ABC-transporter family, enabling the stem cells to export substances like Hoechst 33342 shortly after uptake. Therefore, dye efflux ability can be used as an additional marker for LT-HSCs. In experimental setups, LT-HSCs are mainly sorted as CD34low/- Flt3-KSL cells (low or negative for CD34, Flt3-negative, lineage-negative, Sca-1-positive and c-kit-positive). These cells are enriched for LT-HSCs (approx. 30-50% of the cells are LT-HSCs), having the potential to form all differentiated blood cell types via several progenitor stages and the ability of multi-lineage reconstitution in lethally irradiated mice [10]. In contrast to LT-HSCs, short-term HSCs (ST-HSCs) have already initiated differentiation and lack long-term reconstitution potential in serial transplantation experiments [12–16]. Multi-potent progenitor cells (MPPs) can give rise to all kind of progeny but lack self-renewal potential [9]. MPPs can differentiate into common myeloid and lymphoid progenitors [17, 18], which further differentiate to functional blood cells. It is difficult to distinguish MPPs from ST-HSCs. Gain of Flt3 expression has been proposed as a characteristic marker indicating the loss of self-renewal ability during HSC maturation [19]. Therefore, in addition to CD34, Flt3 represents another useful marker to prospectively isolate LT-HSCs, ST-HSCs and MPPs. Combined use of CD34, Flt3 and KSL markers gives rise to a classical phenotype pattern of long-term HSCs (CD34low/-Flt3-KSL), short-term HSCs (CD34+Flt3-KSL) and multipotent progenitors (CD34+Flt3+KSL). Recently, SLAM (surface lymphocyte activation marker) markers have been discovered as powerful markers for identifying HSCs. By comparing changes in gene expression profiling in highly purified stem cells versus committed progenitor cells, SLAM family members, including CD150, CD48, and CD244, were found to be differentially expressed at different stages of hematopoiesis in a way that associates with self-renewal potential [8]. At the Else Kröner-Fresenius Symposium on the Molecular Mechanisms of Adult Stem Cell Aging, independent lines of evidence came from 2 research groups, headed by Dr. Hiromitsu Nakauchi and Dr. Derrick Rossi, suggesting that CD150 is indeed a powerful marker for isolating most primitive LT-HSCs (see below), thus the most powerful, primitive HSC can be prospectively sorted by CD150high CD34low/-Flt3-KSL phenotype.
Fig. 1. Overview of the hematopoietic hierarchy and cell surface markers characterizing the different progenitor cells. CLP = Common lymphoid progenitor; CMP = common myeloid progenitor; GMP = granulocytic and monocytic progenitor; MEP = megakaryocytic and erythroid progenitor; Ep = erythroid progenitor; Mkp = megakaryocyte progenitor. Reproduced with permission from Weissman et al. [3].
Functional Changes during Aging
During the symposium, Prof. Gerald de Haan discussed current theories of HSC-aging. On the one hand, it is possible that HSCs age uniformly, with the complete stem cell population growing old. This is termed the ‘population aging model’ and for decades was thought to be true. Recently, opinions have changed and the theory of ‘clonal selection’ has been developed. According to this model, only a subset of HSCs undergo cellular aging. These altered cells accumulate over the lifetime, while the subpopulation of young HSCs gets constantly smaller. Different lines of evidence suggest that DNA damage induces checkpoints that may lead to a depletion of the most affected HSCs, although HSCs appear to be more resistant to damage accumulation than are progenitor cells (see chapter 9). It is also possible that different populations of HSCs show different rates of aging (possibly depending on their DNA damage or replication history). According to this model, different sub-populations of HSCs age at different speeds, leading to a selective depletion of some HSC subpopulations and an altered composition of stem cells during aging.
In this context, Christa Müller-Sieburg made an important contribution during the symposium. Her data support the hypothesis of clonal selection of HSC subpopulations during aging and indicate that there are 3 different populations of HSCs: (1) balanced HSCs (Bala); (2) lym-phoidbiased HSCs (Ly-bi), and (3) myeloid-biased HSCs (My-bi). All types of HSC produce myeloid and lymphoid cells in blood; however, Ly-bi HSCs generate more lymphoid cells, and My-bi HSCs generate more myeloid cells than Bala HSCs. Surprisingly, these cells do not show aging phenotypes, as tested in limiting dilution transplantation experiments. However, the relative proportion of these individual subpopulations of HSCs changes during aging and, therefore, different reconstitution capacities of the total pool of HSCs occur. In conclusion, these data suggest that HSCs do not age in terms of losing their repopulation potential, but rather the My-bi HSCs accumulate with aging while the other 2 subpopulations of HSCs decrease, resulting in age-dependent skewing of hemato-lymphopoiesis potential with a decrease in lymphopoiesis and an increase in myelopoiesis. Furthermore, there is experimental evidence indicating that the My-bi HSCs from young mice are similar to those from aged mice regarding myelopoiesis, reduced lymphopoiesis, blunted IL-7 response, and repopulating capacity. The increase of myeloid-biased HSCs is a gene-driven phenotype. DBA2 mice have a shorter lifespan but higher numbers of HSCs at young age compared to C57BL/6J mice (see below). Although both C57Bl/6J and DBA/2 mouse strains show a reduction in Ly-bi HSCs and an increase in My-bi HSCs during aging, the short-lived DBA/2 mouse strain shows an earlier accumulation of My-bi HSCs compared to the C57BL/6J mouse strain. In addition, the increase in myelopoiesis was more severe in aged DBA2 mice (showing 80% My-bi HSCs) compared to aged C57Bl/6J mice (showing up to 60% My-bi HSCs). In summary, the selective survival of My-bi HSCs appears to be the main reason for impaired lymphocyte production in aging wild-type mice [20, 21].
This assumption was recapitulated by Dr. Nakauchi's and Dr. Rossi's experimental data indicating that CD150high CD34low/-KSL cells show myeloid dominant reconstitution, whereas CD150lowCD 34low/-KSL cells showed balanced or lymph-myeloid reconstitution. The CD150high CD34low/-KSL cells have a molecular profile that appears myelo-erythroid primed, while CD150low CD34low/-KSL cells are more lymphoid primed. The greater self-renewal potential of myeloid-biased CD150high CD34low/-KSL cells resulted in their expansion in the primitive stem cell pool during aging. These data support the model of clonal selection of subpopulations of HSCs during aging; however, we cannot conclude that the increase of My-bi HSCs in aged mice is solely due to population shift. In fact, most probably both clonal selection and population shift take place in aged mice. Further study is necessary to make a clear conclusion on this issue.
Although serial transplantation experiments were often used as a standard approach to evaluate stem cell self-renewal, Dr. Irving L. Weissman mentioned that such experiments could artificially be complicated by the effects of irradiation. Dr. Weissman explained that irradiation might create an environment in the host bone marrow that promotes the cell cycle of engrafted HSCs and probably favors the selection of My-bi HSCs. It is known that cycling HSCs do not engraft well, therefore a stochastical loss of cycling HSCs during serial transplantation could occur. In contrast, in Rag-/- mice pre-conditioned with ACK2 (anti c-kit targeting monoclonal antibody), which wipes out the HSCs without irradiation damage, HSCs immediately go back to a low cycling status once they have reconstituted the stem cell pool [22].
The molecular mechanisms that lead to stem cell aging or the selection of subpopulations of stem cells during aging remain to be defined. One possibility is that there is no perfect self-renewal in adult HSCs or in certain subpopulations of HSCs. Taking advantage of the known HSC markers (see above), HSCs were prospectively isolated and purified from C57BL/6J mice for evaluating the impact of aging on the stem cell com...