
eBook - ePub
The Ghrelin System
- 180 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
The Ghrelin System
About this book
The ghrelin story started more than 30 years ago with the discovery of synthetic GH secretagogues. Only in 1999 was ghrelin' a natural GH-releasing peptide, discovered. Ghrelin, however, is much more than simply a natural GH secretagogue. In fact, this hormone is one of the most important factors known for regulating appetite and energy expenditure. Furthermore, ghrelin is the trigger for other neuroendocrine, metabolic and nonendocrine actions. This book, written by researchers who provided the major contributions to our current knowledge of this complex system, gives a comprehensive overview of the recent advances in ghrelin research. The hormone's influence on the cardiovascular, metabolic and gastroenteropancreatic system, hypothalamus-pituitary-adrenal axis, prolactin secretion, thyroid axis, gonadal axis as well as on behavior is discussed in detail. Furthermore, the clinical perspectives for ghrelin-derived therapeutic products are presented. Illustrating the tight inter-relationship between endocrinology, metabolism, cardiovascular disease and internal medicine, this book is essential reading for all scientists interested in appetite control, body weight and energy expenditure, as well as diabetes mellitus and neuroendocrinology.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Benso A, Casanueva FF, Ghigo E, Granata A (eds): The Ghrelin System.
Endocr Dev. Basel, Karger, 2013, vol 25, pp 144-156 (DOI: 10.1159/000346306 )
Endocr Dev. Basel, Karger, 2013, vol 25, pp 144-156 (DOI: 10.1159/000346306 )
______________________
Products of the Ghrelin Gene, the Pancreatic β-Cell and the Adipocyte
Riccarda Granata · Ezio Ghigo
Department of Medical Sciences, Division of Endocrinology, Diabetology and Metabolism, University of Turin, Turin, Italy
______________________
Abstract
The ghrelin system comprises acylated ghrelin (AG), unacylated ghrelin (UAG) and obestatin, besides the receptor for AG, the growth hormone (GH) secretagogue receptor type 1a (GHS-R1a), and the enzyme-promoting ghrelin acylation, ghrelin O-acyltransferase (GOAT). The ghrelin peptides exert a variety of biological actions, including regulation of energy homeostasis and glucose metabolism, as well as survival and proliferative effects in different cell types. Besides the stomach, its main site of production, ghrelin is expressed in pancreatic islets, where it represents an independent islet cell population. AG exerts insulinostatic actions, UAG has been shown to oppose the AG inhibitory effects on insulin secretion, and obestatin has demonstrated insulinotropic activities. Although differences exist in the regulation of glucose homeostasis, all peptides display survival and antiapoptotic actions in pancreatic β-cells, preserving β-cell mass and function both in vitro and in vivo. The ghrelin system is also expressed in adipose tissue, and ghrelin effects have been demonstrated in both white and brown adipocytes. Indeed, AG, UAG and obestatin promote adipogenesis and glucose uptake, and inhibit adipocyte lipolysis. Interestingly, despite similar effects at the cellular level, results from ghrelin, GHS-R and GOAT knockout mice have indicated that AG display diabetogenic effects in vivo. Conversely, UAG and obestatin positively regulate glucose metabolism, and a new role has been recently proposed for obestatin on adipocyte function and insulin sensitivity.
Copyright © 2013 S. Karger AG, Basel
Ghrelin, a 28-amino acid peptide produced by the stomach, was discovered as endogenous ligand for the growth hormone (GH) secretagogue receptor type 1a (GHS-R1a) [1]. Ghrelin potently stimulates GH release and food intake, induces adiposity and exerts a wide range of central and peripheral actions [2]. Ghrelin acylation on the third serine residue, promoted by ghrelin O-acyltransferase (GOAT), is essential for binding to GHS-R1a and for the central activities of the peptide [3-5]. Besides the stomach, acylated ghrelin (AG) and GHS-R1a are produced in different central and peripheral tissues, including the pancreas and adipose tissue [6-10].
Ghrelin peptides exist in two major forms, acylated ghrelin (AG) and unacylated ghrelin (UAG), of which UAG is the most abundant [1, 11]. UAG, like AG, is mainly produced in the stomach; however, it is devoid of binding to GHS-R1a and therefore unable to stimulate GH release and food intake. Nonetheless, UAG exerts many biological activities, some of which similar and other opposed to or independent of AG. Interestingly, AG and UAG display the same effects in different cell types and recognize common binding sites, suggesting the existence of a yet unknown receptor which is bound by both peptides [12-15].
Obestatin was recently discovered as a 23-amino acid amidated peptide encoded by the ghrelin gene [16]. Initially identified as the ligand of the orphan receptor GPR39, obestatin was claimed to oppose ghrelin effects on food intake [16, 17]; however, both findings have been questioned and to date obestatin physiological role has yet to be defined [18, 19]. Like ghrelin, obestatin is produced in the gastrointestinal tract, as well as in central and peripheral tissues [20]. Moreover, it exerts both central and peripheral actions and has been recently found to play a role in pancreatic β-cell survival and function [21], as well as in adipocyte function and glucose metabolism [22-24].
The role of the ghrelin gene-derived peptides in the pancreas and adipose tissue is becoming an important topic, particularly in light of the potential therapeutic applications in conditions such as obesity and insulin resistance. The present review highlights the most recent findings on AG, UAG and obestatin functions in pancreatic β-cells and adipocytes; indeed, these cells play a major role in glucose and lipid metabolism (fig. 1).
Ghrelin System in the Endocrine Pancreas
Ghrelin and GHS-R1a expression have been demonstrated in both human and rodent pancreatic islets [6, 7, 12, 20, 25, 26] and ghrelin in fetal life was found to be more abundant in the pancreas than in the stomach, suggesting a paracrine/autocrine role of the ghrelin system in hormonal secretion and/or islet growth and differentiation [27, 28]. Interestingly, ghrelin-producing ε-cells have been identified in pancreatic islets, and during fetal development ε-cells showed an ontogenetic and morphogenetic pattern distinct from that of glucagon-producing α-cells and insulin-producing β-cells [28-30].
In the developing mouse and rat pancreas, ghrelin immunoreactivity can be detected as early as embryonic day E9.5 and E15, respectively, and remains positive throughout gestation, peaking just before birth [30, 31]. Notably, ε-cells replaced β-cells and most of α-cells in two mouse models of pancreas development, suggesting a relationship between ghrelin-, insulin- and glucagon-producing cells in the developing pancreas [31, 32]. In normal adult rats, ghrelin immunoreactivity has been observed in the peripheral region, whereas in diabetic rats, it was found in the central region of the islets [33].
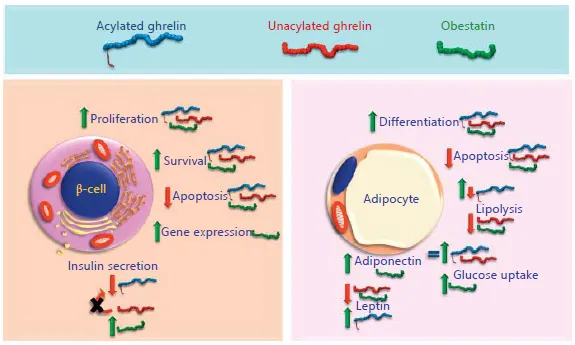
Fig. 1. Effects of the ghrelin gene-derived peptides in the pancreatic β-cell and the adipocyte. Acylated ghrelin (blue), unacylated ghrelin (red) and obestatin (green) exert both similar and opposed actions. Only the most important effects are indicated and in some cases, such as gene expression (regulation of genes involved in β-cell survival, differentiation and function, left panel), data are obtained from studies performed with only one of the three peptides.
In humans, ε-cells have been detected from midgestation to early postnatal period, accounting for approximately 10% of all islet cells [28, 29]. The developmental pattern of the ghrelin cells in human pancreas, although clearly distinct, was found to be quite similar to that of glucagon-expressing cells. Indeed, in fetal pancreas, ε- and α-cells reside at the periphery of the islets, whereas β-cells are mostly located in the islet core [26, 34-36]. Interestingly, colocalization of ghrelin and glucagon has been shown in both human and rat pancreas, which further supports the possible developmental link between these two cell types [6, 30]. Recently, ghrelin immunostaining was found to be either dependent or independent of glucagon, suggesting the existence of two different pancreatic ghrelin cell populations [37].
With regard to GHS-R1a, its immunoreactivity in human pancreatic islets partially overlaps with that of insulin in β-cells, in agreement with the many findings on β-cell responsiveness to ghrelin stimulation [12, 38]. Moreover, like ghrelin, GHS-R1a is expressed in human and rat developing pancreatic islets, from early gestation to adult [12, 27, 39].
Obestatin colocalizes with ghrelin at the periphery of the islets in rat, as well as in fetal and adult human pancreas [20, 21, 37, 40, 41]. Furthermore, both human pancreatic islets and INS-1E β-cells were found able to release obestatin, suggesting autocrine/paracrine effects of the peptide [21]. Besides the different ghrelin gene products, high levels of GOAT mRNA have been found in both stomach and pancreas [3], indicating regulatory mechanisms controlling AG versus UAG production and the consequent effects of these peptides on glucose metabolism and/or cell proliferation and survival.
Ghrelin Gene-Derived Peptides and the Pancreatic β-Cell
Different studies have shown that AG, UAG and obestatin play key roles in pancreatic β-cells and human pancreatic islets [38, 42]. We will not describe here their effects on insulin secretion and glucose metabolism, as they will be thoroughly covered in the chapter by Dezaki et al. [this vol.]. We will focus instead on β-cell proliferation and survival, and the underlying signaling pathways triggered by the peptides.
The survival and antiapoptotic effects of the ghrelin gene products have been described in a variety of cell types [43]. Interestingly, AG and UAG, when investigated together, showed similar ability to increase cell proliferation and survival and to inhibit apoptosis. Due to UAG inability to bind to GHSR-1a, it was proposed the existence of an alternative receptor involved in these effects [12, 13, 15, 44].
In HIT-T15 and INS-1E β-cells, as well as in human pancreatic is...
Table of contents
- Cover Page
- Front Matter
- Ghrelin Discovery: A Decade After
- The Ghrelin Receptors (GHS-R1a and GHS-R1b)
- Discovery of Ghrelin O-Acyltransferase
- Genetics of the Ghrelin System
- Ghrelin and the Gut
- Ghrelin as a GH-Releasing Factor
- Other than Growth Hormone Neuroendocrine Actions of Ghrelin
- Ghrelin, the Gonadal Axis and the Onset of Puberty
- Ghrelin and the Cardiovascular System
- Ghrelin - A Key Pleiotropic Hormone-Regulating Systemic Energy Metabolism
- Ghrelin, Reward and Motivation
- Des-Acyl Ghrelin: A Metabolically Active Peptide
- Ghrelin and Tumors
- Ghrelin Function in Insulin Release and Glucose Metabolism
- Products of the Ghrelin Gene, the Pancreatic β-Cell and the Adipocyte
- Clinical Perspectives for Ghrelin-Derived Therapeutic Products
- Author Index
- Subject Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access The Ghrelin System by A. Benso,F. F. Casanueva,E. Ghigo,R. Granata,A., Benso,F.F., Casanueva,E., Ghigo,R., Granata, A. Benso, F. F. Casanueva, E. Ghigo, R. Granata in PDF and/or ePUB format, as well as other popular books in Medicine & Cardiology. We have over one million books available in our catalogue for you to explore.