- 128 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
The discovery of proton pump inhibitors (PPIs) and their development over the years has dramatically changed the management of acid-related diseases. Today, the therapeutic domain of PPIs ranges from relief of symptoms to cure of mucosal lesions in the upper gastrointestinal tract. PPIs are among the most widely sold drugs in the world and are now even available as over-the-counter medication. This publication presents the experience of the last 25 years during which PPIs have become of enormous value in gastroenterology. The authors provide an update on a variety of subjects, starting with an introduction to the discovery and development of PPIs. This is followed by chapters on pharmacokinetics, pharmacodynamics and pharmacogenetics, gastroesophageal reflux disease, gastroprotection, Helicobacter pylori eradication treatment, peptic ulcer disease, functional dyspepsia, acid suppression in exocrine pancreatic insufficiency, and gastrointestinal and systemic side effects. Readers who are interested in a current overview of PPIs and their various applications will find this book of great value.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Proton Pump Inhibitors: A Balanced View by T. Chiba,P. Malfertheiner,H. Satoh,T., Chiba,P., Malfertheiner,H., Satoh, G. Rogler,G., Rogler in PDF and/or ePUB format, as well as other popular books in Medicine & Pharmacology. We have over one million books available in our catalogue for you to explore.
Information
Chiba T, Malfertheiner P, Satoh H (eds): Proton Pump Inhibitors: A Balanced View.
Front Gastrointest Res. Basel, Karger, 2013, vol 32, pp 1-17 (DOI: 10.1159/000350624)
Front Gastrointest Res. Basel, Karger, 2013, vol 32, pp 1-17 (DOI: 10.1159/000350624)
______________________
Discovery and Development of Proton Pump Inhibitors
Hiroshi Satoh
Department of Pharmacology and Experimental Therapeutics, Division of Pathological Sciences, Kyoto Pharmaceutical University, Kyoto, Japan
______________________
Abstract
Omeprazole (OPZ), developed by AB Hässle/Astra (Sweden), and lansoprazole (LPZ), developed by Takeda Chemical Industries Ltd. (Japan), were the world's first and second proton pump inhibitors (PPIs), respectively, approved for clinical use in humans. These PPIs have been widely used for more than 20 years in the treatment of acid-related diseases such as gastroduodenal ulcers and reflux esophagitis. In the process of discovery of the PPIs, both companies independently identified the same compound (2-[(2-pyridylmethyl)sulfinyl]-1H-benzimidazole, timoprazole), which had strong antisecretory and antiulcer activities. LPZ and OPZ are derivatives of timoprazole; the LPZ molecule has a trifluoroethoxy group that seems to confer strong antiulcer properties in addition to the compound's antisecretory action. For example, the antisecretory effect of LPZ in rats was approximately twice as strong as that of OPZ, but the antiulcer effects were more than 10 times stronger than those of OPZ in rat models of reflux esophagitis, gastric antral ulcers, and duodenal ulcers. Furthermore, LPZ, but not OPZ, also prevented the formation of small intestinal lesions induced by NSAIDs in rats. These effects can be explained, at least in part, by the finding that LPZ not only has antisecretory activity but also mucosal protective activity in which both capsaicin-sensitive sensory neurons and nitric oxide are involved. It has also been reported that LPZ has highly specific antibacterial effects on Helicobacter pylori. Both OPZ and LPZ are racemates, each having two optical isomers (S and R). Both the S-isomer of OPZ (esomeprazole) and the R-isomer of LPZ (dexlansoprazole) have been developed as second-generation PPIs with enhanced bioavailability and antisecretory activities in humans. This chapter reviews the history of the discovery and development of PPIs, and discusses the unique pharmacological properties of LPZ independent from the compound's antisecretory activity.
Copyright © 2013 S. Karger AG, Basel
Peptic ulcers, such as gastric and duodenal ulcers, had long been a significant and intractable condition in humans until the discovery of the histamine H2-receptor antagonist (H2-RA) cimetidine in the late 1970s, which led to dramatic improvement in treatment. Thereafter, many H2-RAs, such as ranitidine and famotidine, were developed and have since been used effectively for the treatment of peptic ulcers. H2-RAs are very useful drugs, but they also have several drawbacks, i.e. they effectively inhibit basal gastric acid secretion at nighttime but are less effective for inhibition of acid secretion induced by various stimuli such as stress and meals during the day, they are not always effective for the treatment of gastroesophageal reflux disease (GERD) and Zollinger-Ellison syndrome, and their efficacy is reduced after repeated administration. Some of these problems were resolved by the discovery of the proton pump inhibitors (PPIs), such as omeprazole (OPZ; AB Hassle/Astra, Sweden) and lansoprazole (LPZ; Takeda Chemical Industries Ltd., Japan). The inhibitory effects of PPIs on gastric acid secretion are more prolonged than those of H2-RAs, and the drugs have significantly improved the outcome of treatment for acid-related diseases. In particular, the efficacy for the treatment of reflux esophagitis and eradication of Helicobacter pylori with PPIs are significantly greater than with H2-RAs. In this chapter, both a brief history of the discovery and development of the PPIs and the unique pharmacological properties of LPZ independent from its antisecretory action are reviewed.
The author worked for Takeda Chemical Industries, Ltd. from 1969 to 1997, and was deeply involved in the discovery and development of lansoprazole.
The Discovery of Proton Pump Inhibitors
Pharmaceutical companies often synthesize new compounds through the modification of existing compounds and subsequent evaluation of their pharmacological activities. In some cases, different pharmaceutical companies working independently may coincidentally end up synthesizing the same compound as a new drug candidate. This was in fact the case with 2-pyridylthioacetamide and timoprazole, the discovery of which played a key role in the development of LPZ and OPZ.
Discovery of 2-Pyridylthioacetamide (AG-35, CMN 131)
During initial efforts to screen for antiulcer drugs with antisecretory activity, Takeda found that 2-pyridylthioacetamide (coded by Takeda as AG-35) and its analogs had strong antisecretory and antiulcer activities [1], and in 1969 the company applied for a Japanese patent for the compounds as novel antiulcer drugs (fig. 1) [2]. In 1971, the French pharmaceutical company Servier also reported strong antisecretory activities of thioamide derivatives including AG-35 (coded by Servier as CMN 131; fig. 1) [3]. However, these compounds were not developed as new drugs, mostly due to the demonstration of acute toxicity in animals. According to a review by Olbe et al. [4], the antisecretory activity of CMN 131 reported by Servier prompted AB Hassle to initiate research in an attempt to identify structural analogs of CMN 131 with strong antisecretory activity but minimal toxicity. Fig. 1 shows the milestones in the development of PPIs by Takeda and AB Hassle. Interestingly, both companies began their research on antisecretory and antiulcer drugs with the same compound (AG-35 and CMN-131).
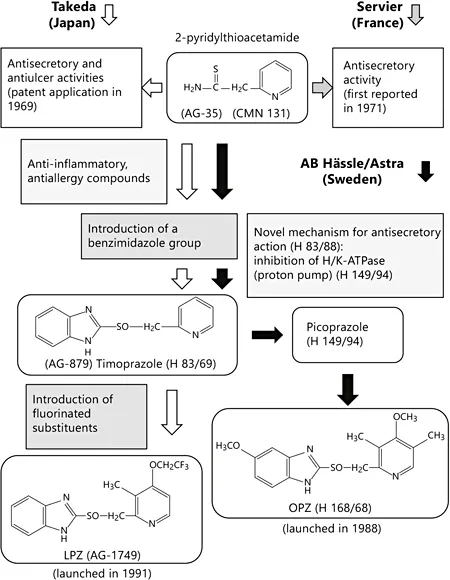
Fig. 1. Discovery and development of the PPIs LPZ and OPZ. Two compounds, 2-pyridylthioacetamide and timoprazole, played an important role in the process of discovery of LPZ (Takeda Chemical Industries Ltd.) and OPZ (AB Hassle/Astra).
Discovery of Timoprazole (AG-879, H 83/69)
The next stage in the evolution of PPIs was the identification of the compound timoprazole. AB Hassle was initially interested in CMN 131 because the drug was found to have strong antisecretory activity, although it had undesired acute toxicity. Company scientists speculated that the toxicity of CMN 131 might depend on the thioamide group in the molecule's structure. They synthesized many derivatives of CMN 131 and found that compound H 124/26, in which the thioamide group of CMN 131 was eliminated and a benzimidazole ring was incorporated, had powerful antisecretory activity but no acute toxicity. However, due to a conflicting preexisting patent, H 83/69 (a sulfoxide derivative of H 124/26, timoprazole) was selected instead for further development [4]. Subsequent long-term toxicological studies of timoprazole revealed that H 124/26 caused enlargement of the thyroid gland. Other derivatives were then screened and the compound H 149/94 (picoprazole; fig. 1) was found to have strong antisecretory activity without producing adverse effects on the thyroid/thymus. The company also reported that a derivative with a substituted benzimidazole group (coded by AB Hassle as H 83/88) inhibited acid secretion stimulated by dibutyryl-cAMP in isolated guinea pig gastric mucosa, i.e. the mode of action was quite different from that of H2-RAs [5]. They also found that H 149/94 inhibited H+, K+-ATPase, which is localized in gastric parietal cells and serves as a proton pump to produce gastric acid [6]. This finding was critical for the development of the PPIs. AB Hassle eventually developed OPZ (H 168/68) as the world's first PPI (fig. 1).
From the late 1970s to early 1980s, Takeda continued to search for new antiulcer drugs with antisecretory activity. During that time ranitidine had already been approved as the second commercially available H2-RA and, therefore, Takeda considered that additional research to develop other new H2-RAs would not be commercially viable. However, AB Hassle's findings regarding novel mechanisms for the inhibition of acid secretion stimulated Takeda to screen for antisecretory drugs with a different mode of action from that of H2-RAs. Through its own independent research efforts, Takeda coincidentally identified the same compound as H 83/69 – coded by Takeda as AG-879 – from a series of newly synthesized compounds that were screened for anti-inflammatory and antiallergy effects, and it was found to have strong antisecretory and antiulcer activity in rats. However, it was subsequently learned that this was the same compound as timoprazole.
Introduction of Fluorinated Substituents to Timoprazole
Takeda randomly screened more than 700 compounds in an attempt to identify antisecretory compounds with new basic chemical structures that differed from timoprazole, but Takeda eventually recognized that the basic structure of timoprazole was essential for PPI activity. Takeda investigated various modifications of timoprazole and eventually found that introduction of fluorinated substituents, such as a trifluoroethoxy group, to timoprazole (AG-879) markedly improved the antiulcer properties of the compound [7]. While human gastric ulcers are often observed in the antral area of the stomach, drug development research for this condition had been hampered by the lack of a suitable animal model for gastric antral ulcers; however, we reported that indomethacin (IND) given before or after a 1-hour feeding in rats fasted previously for 24 h caused ulcers in the antral area of the stomach [8]. The effects of fluorinated compounds were then examined in this antral ulcer model. As shown in table 1, fluorinated compounds, including LPZ, exhibited markedly stronger antiulcer activities compared with nonfluorinated reference compounds in this model [9]. The effect of LPZ was further examined in a mepirizole-induced duodenal ulc...
Table of contents
- Cover Page
- Front Matter
- Discovery and Development of Proton Pump Inhibitors
- Pharmacokinetics, Pharmacodynamics, and Pharmacogenetics of Proton Pump Inhibitors
- Proton Pump Inhibitors in Gastroesophageal Reflux Disease
- Proton Pump Inhibitors in Gastroprotection: Prevention and Healing of Nonsteroidal Anti-Inflammatory Drug-, Aspirin-, and Cyclooxygenase-2 Inhibitor-Induced Gastroduodenal Lesions
- Proton Pump Inhibitors: Key Ingredients in Helicobacter pylori Eradication Treatment
- Proton Pump Inhibitor Management in Bleeding Peptic Ulcer Disease
- Proton Pump Inhibitors in Functional Dyspepsia
- Role of Acid Suppression in Exocrine Pancreatic Insufficiency: A Therapeutic Principle
- Proton Pump Inhibitors and Gastrointestinal Side Effects
- Administration of Proton Pump Inhibitors and Risk of Systemic Side Effects
- Author Index
- Subject Index