![]()
Marone G, Granata F (eds): Angiogenesis, Lymphangiogenesis and Clinical Implications.
Chem Immunol Allergy. Basel, Karger, 2014, vol 99, pp 37-70 (DOI: 10.1159/000354169)
______________________
Neuropilins: Role in Signalling, Angiogenesis and Disease
Ian Zachary
Centre for Cardiovascular Science and Medicine, Division of Medicine, University College London, London, UK
______________________
Abstract
Neuropilins (NRPs) are co-receptors for class 3 semaphorins and for members of the vascular endothelial growth factor (VEGF) family of angiogenic cytokines. Genetic analysis of the role of NRPs in mice shows that NRP1 is essential for embryonic neuronal pathfinding and cardiovascular development, mediated via semaphorins and VEGF, respectively, while NRP2 has a more restricted role in neuronal patterning and lymphangiogenesis. NRPs are thought to mediate functional responses, most importantly cell migration, as a result of complex formation with other receptors, such as plexins in the case of semaphorins and the VEGF receptor, VEGFR2, resulting in enhanced signalling via some intracellular pathways. Recent findings indicate that NRPs may have important biological roles in other physiological and disease-related processes. In particular, NRPs are highly expressed in diverse tumour cell lines and human neoplasms and have been implicated in several biological processes regulating tumour growth in vivo, suggesting that NRP1 may be a future therapeutic target in cancer.
Copyright © 2014 S. Karger AG, Basel
Neuropilin-1 (NRP1) was discovered as the antigen recognised by the A5 monoclonal antibody, which localised in the tadpoles of Xenopus laevis to the neuropile, a superficial layer comprising a dense network of glial cells, synapses, axons, dendrites and neurons [1-3]. The subsequent generation of NRP1 mutants in mice and zebrafish established an essential role of this molecule in embryonic development of the vertebrate nervous and cardiovascular systems [4-7]. A key feature of NRP1 and the structurally related molecule, NRP2, is that they function as receptors for two unrelated types of secreted polypeptide ligand: class 3 semaphorins, a family of molecules with key roles in axonal guidance, and several members of the vascular endothelial growth factor (VEGF) family of angiogenic cytokines. In both cases NRP1 is important for regulating cell migration, but causes chemorepulsion in response to semaphorins, and chemotaxis in response to VEGF-A. However, despite the wealth of data regarding the developmental functions of NRPs, the cellular functions of NRPs are not entirely clear, and much uncertainty remains regarding the mechanisms through which NRPs mediate the biological effects of their ligands. Moreover, recent findings in diverse physiological and pathophysiological settings have greatly expanded the repertoire of biological processes in which NRPs potentially play an important role [8-10]. This chapter will review NRP biology with a focus on recent advances in understanding the role of NRPs in angiogenesis, in cell signalling and in disease.
Neuropilin Structure
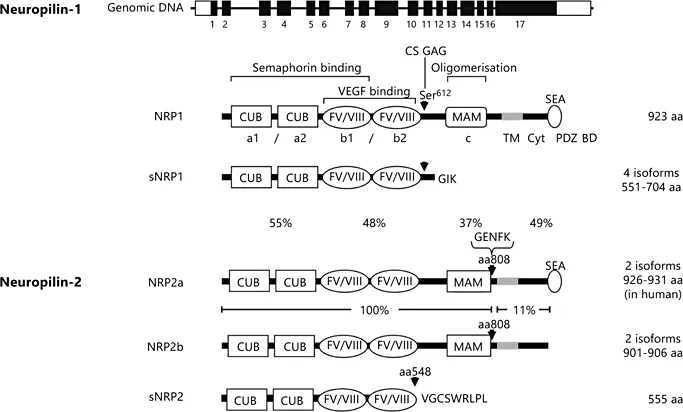
NRP1 and NRP2 are transmembrane glycoproteins of up to 923 and 926 amino acids, respectively, sharing a similar domain structure and an overall amino acid homology of 44% [10, 11]. NRPs comprise large extracellular regions containing two CUB (a1/a2) domains, sharing homology with the complement-binding factors C1s/C1r, sea urchin fibropellins (called Uegf) and bone morphogenetic protein 1, tandem factor V/VIII homology (b1/b2) domains with homology to the C-terminal (C1/C2) domains of blood coagulation factors V and VIII [2], and a MAM (c) domain, a 170 amino acid region, also found in the extracellular domains of functionally diverse proteins, including meprin (a cell surface glycoprotein), A5 antigen, and receptor tyrosine protein phosphatase μ (MAM). NRPs have a single transmembrane domain and small cytoplasmic domains of 44 amino acids in NRP1 and 43 in NRP2 (fig. 1). The carboxy-terminal three amino acids, SEA, present in NRP1 and the NRP2a isoform, form a consensus PDZ domain binding motif, which mediates association with a PDZ domain protein called neuropilin-interacting protein-1 (NIP1), synectin or RGS-GAIP-interacting protein (GIPC) [12].
An important role of the extracellular region is ligand binding. The a1/a2 domains are essential for the binding of semaphorins, while the b1 domain is required for VEGFA165 binding with the b2 domain also necessary for optimal VEGF-A165 binding, and the b1/b2 domains are also important for semaphorin binding. The crystal structure of the NRP1 b1 domain displays a similarity to the three-dimensional structure of the FV/VIII C2 domain [13]. Crystal structures of the NRP1 b1 domain bound either to the peptide ligand, Tuftsin [14], to antibodies directed against either the VEGF or Sema3A binding domains [15], or to small molecule ligand mimetics [16], have identified the VEGF-binding pocket and the key residues required for VEGF binding. The c or MAM domain has homology to other MAM domains present in diverse proteins that are thought to mediate homophilic protein-protein associations important for homodimerisation or oligomerisation, and have also been implicated in regulating protein stability. The MAM domain is thought to play a role in NRP-1 oligomerisation [17, 18], though the function of this region of NRP1 is still poorly understood.
NRP1 is a glycoprotein though the degree of glycosylation appears to vary between different cell types. In several tumour cell lines and in cultured vascular smooth muscle cells (VSMC) NRP1 also occurs as a high-molecular-weight glycosylated species of >250 kDa in addition to the major species of ~ 130 kDa corresponding to the fulllength protein, and this modified form appears to be expressed at a lower level in cultured endothelial cells [19-21]. NRP1 is modified by addition of an O-linked heparan sulphate and/or chondroitin sulphate glycosaminoglycan (GAG) moiety preferentially to serine 612 in the linker region between the b2 and MAM domains (fig. 1). In contrast, there is no evidence for a high-molecular-weight GAG modified form of NRP2. NRP1 and NRP2 are also modified by asparagine(N)-linked glycosylation as indicated by the effect of tunicamycin inhibition of N-glycosylation [22]. CS-GAG modification is reported to enhance both VEGF binding to neuropilin and cell survival, and to downregulate VEGFR2 expression levels in vascular smooth muscle cells [19]. Further studies are needed to fully determine how glycosylation alters the ligand binding and other functional properties of NRPs.
Fig. 1. Neuropilin gene organisation and protein structure. The NRP1 gene comprises 17 exons (black rectangles), encoding a full-length protein of 923 amino acids (aa) consisting of an extracellular region with two tandem CUB (a1 and a2) domains (complement-binding factors C1s/C1r, Uegf, bone morphogenetic protein 1), two tandem FV/VIII homology (b1 and b2) domains, a linker region and one MAM (c) domain (meprin, A5 antigen, receptor tyrosine phosphatase μ); a single transmembrane domain (TM), and a 44 aa cytoplasmic (Cyt) domain containing a C-terminal PDZ (post-synaptic density protein (PSD95), Drosophila disc large tumour suppressor (DlgA), and zonula occuldens-1 protein) binding domain motif with the sequence SerGluAla (SEA). Locations of binding sites for semaphorins and VEGF, and the putative oligomerisation domain are indicated. Arrowheads indicate the positions of Ser 612 in NRP1, the major site of O-linked chondroitin sulphate glycosaminoglycan (CS-GAG) modification, insertion at residue 808 in NRP2 of five amino acids GlyGluAspPheLys (GENFK), and addition of 9 amino acids ValGlyCysSerTrpArgLeuProLeu (VGCSWRLPL) following truncation of the b2 domain at residue 548 in soluble NRP2 (sNRP2). Soluble NRP1 (sNRP1) is truncated at the linker region between the second FV/VIII and the MAM domain and ends with the 3 amino acids GlyIsoLys (GIK). Note that not all sNRP1 isoforms contain Ser 612, and are not all modifiable by CS-GAG addition. Percentage amino acid homologies between the domains of full-length NRP1 and NRP2a isoforms (a1/a2, b1/b2, MAM, and intracellular domains, respectively, left to right) and between the NRP2a and NRP2b isoforms (extracellular region, and transmembrane domain plus intracellular domain, respectively) are indicated.
Neuropilin Ligands
NRPs have the ability to bind with high affinity two structurally unrelated classes of ligands with distinct biological functions, the class 3 semaphorins and several members of the VEGF family. These ligand interactions are summarised in figure 2 .
Semaphorins
The class 3 semaphorins are secreted proteins with essential roles in the axonal guidance and homing of sensory neurons, most of which require NRP1 or 2 as obligate co-receptors [23-25]. The major semaphorin ligand for NRP1 is Sema3A (also termed collapsin-1), which induces growth cone collapse in subsets of sensory and sympathetic neurons, including the dorsal root ganglia, and is essential for neurogenesis [26]. NRP1 also binds other secreted class 3 semaphorins with lower affinity, though the biological roles of these interactions are less clear [10]. The best characterized semaphorin ligand for NRP2 is Sema 3F, and Semas 3B, 3C and 3G, but not Sema3A, also bind NRP2 [10].
VEGFs
Vascular endothelial growth factor (VEGF or...