![]()
Sexual
Development
| Sex Dev 2010;4:199–212
DOI: 10.1159/000317090 | Published online: July 27, 2010 |
Ontogenesis of Testis Development and Function in Humans
J.B. Stukenborga E. Colóna,b O. Södera
aPaediatric Endocrinology Unit, Department of Women’s and Children’s Health and bDepartment of Clinical Pathology and Cytology, Karolinska Institutet and University Hospital, Stockholm, Sweden
________________
Key Words
Germ cells · Human spermatogenesis · Somatic cells
________________
Abstract
Functional gonads are mandatory for sexual reproduction and survival of higher animal species. However, at the level of the individual subject, acquired or inherited gonadal dysfunction and infertility are not commonly associated with severe life-threatening phenotypes. Medical progress and increased societal interest have led to more prioritised agendas for reproductive health problems. Increasing attention is focused on disorders of sex development, fertility and sexual function. Despite this engagement, our understanding of the detailed molecular and cellular adverse events behind such problems is still incomplete. Critical early steps, such as determination of the gonads, occur at precise temporal windows of development. The sex chromosomes are obvious critical contributors, but many other human chromosomes also contribute to sex differentiation, engaging multiple genes and proteins. The aim of this review is to give an up-to-date and comprehensive summary of the events required for gonadal ontogenesis in the human male, from the stage of embryonic sex determination to postnatal maturation including puberty. The principal genes involved in these processes are tabulated and discussed. Morphological events relevant for human gonadal development are covered, in particular in connection with early germ cell maturation and spermatogenesis. Consequences of maldevelopment caused by, e.g. cryptorchidism, are discussed.
Copyright © 2010 S. Karger AG, Basel
Introduction
In humans, the important first steps of sexual differentiation occur during the initial 7 weeks of embryonic development and appear as several successive events starting with establishment of the genetic sex, development of the gonadal ridge and immigration of primordial germ cells followed by a sexually dimorphic differentiation of the gonadal anlagen into either testes or ovaries. Until this point of time, referred to as the indifferent stage of gonadal development, no morphologically distinct sex differences can be noticed in developing human gonads. One critical event in sex differentiation is the determination of the gonads. This developmental phase establishes the hormonal dimorphism which, in turn, has a major impact on several later events of the male as well as female paths. This review will deal with differentiation of the male gonad covering its maturation from its first appearance to the pubertal activation. Recent discoveries added to increase the knowledge of testicular ontogenesis together with the growing list of genes involved in this process will be presented and discussed.
Table 1. Chronology of important early events in human male sex differentiation
Event | Age at start (dpc) | Size CRL (mm) |
Genetic sex | 0 | |
PGC migration from yolk sac | 28 | 4 |
Formation of gonadal ridge | 32 | 5 |
PGCs reach gonadal ridge | 37 | 10 |
Male sex determination | 42 | 15 |
Leydig cells appear | 55 | 30 |
Androgen, INSL3 detectable | 63 | 40 |
Testicular descent (1st phase) | 64 | |
dpc = Days post conception; CRL = crown rump length (‘sitting height’). |
The Primitive Gonad
By day 32 post conception (pc), the gonadal anlagen can be recognised as paired bipotential structures in the developing human embryo. They are situated at the ventromedial surface of the mesonephros and appear from the mesoderm by contributions from somatic mesenchymal cells from the mesonephros and epithelial cells migrating from the coelomic surface of the gonadal ridge. As mentioned before, no sexual dimorphism can be distinguished morphologically at this stage of development. Primordial germ cells (PGCs), which become gonocytes later on, cannot be observed at this early time of gonadal formation [Shawlot and Behringer, 1995; Torres et al., 1995; Miyamoto et al., 1997; Birk et al., 2000; Failli et al., 2000; Park and Jamieson, 2005]. The temporal scale of the important early events of human gonadal differentiation is displayed in table 1.
The mesonephros also constitutes the primordium of the adrenal glands and the urinary system. Disruption by gene targeting of any of several involved transcription factors (online supplementary table 1; see www.karger.com/doi/10.1159/000317090) during genital ridge development results at all times in severely affected phenotypes with multiple malformations of the urogenital tract, adrenals and other structures. Therefore, a number of peptide growth factors have been compromised in gonadal development from the indifferent gonadal anlagen, most notably the insulin-like growth factor (IGF) superfamily [Nef et al., 2003]. The formation of the urogenital ridge is mainly controlled by 2 transcriptional regulators: the tumour suppressor gene Wilms’ tumour-associated gene-1 (WT1) and the orphan nuclear receptor steroidogenic factor-1 (SF1). WT1 is a DNA- and RNA-binding protein with transcriptional and posttranscriptional regulation capacity. It is expressed in gonadal stromal, coelomic epithelial cells and immature Sertoli cells, interacts with the cAMP-responsive element-binding protein CITED 2 and is regulated by ‘paired box gene 2’ (PAX2) (see online suppl. table 1). In rodents, disruption of Wt1 leads to lack of formation of kidneys, gonads and adrenals. Distinct but not identical phenotypes can be observed after WT1 loss-of-function (LOF) mutation in humans, resulting in pseudoher-maphroditism and/or urogenital and other malformations in boys with WAGR, Deny-Drash or Frasier syndromes [Pritchard-Jones et al., 1990; Park and Jamieson, 2005; online suppl. table 1]. SF1, expressed in gonadal ridges, is a transcriptional regulator of steroid hydrolases, gonadotropins and aromatase and involved in the stabilisation of intermediate mesoderm, follicle development and ovulation (online suppl. table 1). Additionally, SF1 regulates the anti-müllerian hormone (AMH), dosage-sensitive sex reversal-congenital adrenal hypoplasia critical region on the X-chromosome protein 1 (DAX1) and steroidogenic acute regulatory protein (StAR). These genes are expressed in the somatic testicular compartment and important for normal testicular cord formation, Leydig and Sertoli cell differentiation as well as the initial step of steroidogenesis (online suppl. table 1). Therefore, deletion of Sf1 in mice results in failure of gonadal and adrenal development, whereas the corresponding LOF mutation in humans has a less prominent gonadal phenotype and adrenal insufficiency [Biason-Lauber and Schoenle, 2000; Achermann et al., 2002; Park and Jamieson, 2005; online suppl. table 1]. For a comprehensive account of more genes involved in gonadal formation, please consult online suppl. table 1.
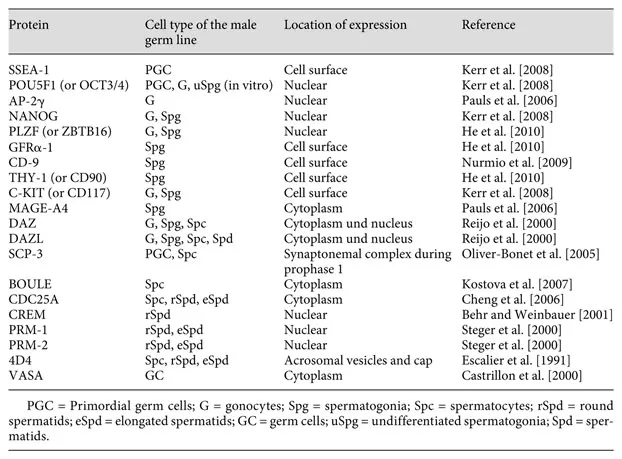
Table 2. Selection of protein expression patterns specific for different male germ cell types in the human testis
Primordial Germ Cells
By the end of the 5th week pc of human embryonic development, 3 different lineages of somatic cell types with bipotential fate, dependent on their future paths (see below), are forming the gonadal anlagen. At this stage, immigrating PGCs are colonising the gonadal structures. After they have permanently been situated in the gonad, they are specified as gonocytes. The PGCs differentiate from epiblast-derived stem cells in the yolk sac. Due to their expression of molecular and cellular markers for pluripotency or early germ cells such as alkaline phosphatase, OCT3/4 and c-kit, they can be distinguished from other cells within the forming gonad (see also table 2 and online suppl. table 1). Guided by extracellular matrix proteins expressed along the dorsal mesentery of the hind gut, the PGCs migrate to the gonadal ridges. During this phase, PGCs exhibit active mitotic proliferation and have expanded in numbers while reaching the gonadal anlagen [Bendel-Stenzel et al., 1998; Wylie, 1999]. In the early testis shortly after determination, gonocytes will continue their mitotic proliferation and then become mitotically quiescent. They will not be recruited into meiosis until much later in time. The decision to enter into meiosis or not is thought to be governed by somatic cells in the male gonad since XY PGCs residing in an ovary follow the female path [McLaren and Southee, 1997]. However, meiotic entry might also be activated by mechanisms intrinsic to germ cells [Morelli and Cohen, 2005]. In males, the absence of germ cells still allows differentiation of somatic cells including Leydig cells with steroidogenic activity. Affected males will undergo pubertal development but are infertile due to a Sertoli-cell-only syndrome.
Somatic Cell Lineages in the Male Gonad
At the end of the 6th week pc of human embryonic development, the indifferent gonad consists of 4 different cells lineages, including gonocytes, with predefined maturational paths dependent on the sex. The crucial somatic cell lineages are Sertoli cells, Leydi...